, Islam A. El-Sayes1 and Sylvia R. Weiner2
(1)
Department for Surgery of Obesity and Metabolic Disorders (Center of Excellence), Sana Klinikum Offenbach, Starkenburgring 66, 63069 Offenbach am Main, Germany
(2)
Department of general and visceral and minimally invasive surgery, Nordwest Hospital, Steinbacher Hohl 2–26, 60488 Frankfurt am Main, Germany
Abstract
The worldwide incidence of obesity is showing an evident increase in the number of obese population from year to year. This rise is not anymore restricted to developed countries. Surgical solutions designed to counteract the obesity epidemic are rapidly increasing, and recent approaches start to replace the already established operations. Distinguished from other procedures, sleeve gastrectomy seems to be the new leading procedure in the near future. This is attributed to many factors, including relative simplicity of the procedure, lower cost, reasonable outcome in terms of weight loss and improvement of the associated comorbidities, and the available conversion options in case of unsatisfactory outcome. Additionally, an accepted postoperative complication rate played a significant role in its widespread. Another procedure is the mini-gastric bypass which can be applied either as a primary procedure or as a secondary solution after a failed sleeve gastrectomy. Other procedures involving sleeve gastrectomy as a restrictive component include, for example, Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy (SADI-S) which avoids the risk of biliary reflux encountered with mini gastric bypass. Additionally, it is a simple procedure compared to the classical duodenal switch operation. Another example is the transit bipartition which saves the pyloric function and the duodeno-jejunal protein absorptive effect, and provides at the same time a distal malabsorptive component, thereby achieving the intended weight reducing and metabolic outcome. Jejuno-ileal and duodeno-ileal interposition seem also to add a significant entero-hormonal effect to the constructed sleeve. EndoBarrier is also a recent minimally invasive procedure which produces a reasonable anti-diabetic outcome. Similarly, different trials for recent endoscopic approaches are currently in the evaluation phase. Added to this, several gastric space occupying modalities have also been tried.
3.1 Introduction
In 2005, 23.2 % (937 million) of the world’s adult population was overweight, and 9.8 % (396 million) was obese. According to current projections, the number of overweight individuals will increase from 2005 to 2030 by 44 %, to reach a total of 2.16 billion, corresponding to 38 % of the world’s adult population (Kelly et al. 2008). During the same interval, the number of obese individuals will increase by 45 % to reach a total of 1.12 billion (20 % of the world’s adult population).
In 2005, the prevalence of overweight was higher in economically developed countries compared to developing countries (35.2 vs 19.6 %). Similarly, the prevalence of obesity was higher in developed countries compared to developing ones (20.3 vs 6.7 %). However, growth in population size, aging, urbanization of lifestyle all will contribute to an epidemic of overweight and obesity in developing regions in the next few decades (Kelly et al. 2008).
This overwhelming increase in the prevalence of morbid obesity with its associated comorbidities pushed institutions worldwide to search for new minimally invasive modalities to face this epidemic. Over the past years, standard procedures like biliopancreatic diversion (BPD), duodenal switch (DS), gastric banding (LAGB), and Roux-en-Y gastric bypass (RYGB) established their position in the armamentarium of bariatric procedures. More recently, new surgical as well as endoscopic bariatric procedures started to replace, or at least compete with these standard procedures. In this chapter, we will focus on the evolution of sleeve gastrectomy (SG), mini gastric bypass (MGB), as well as other bariatric procedures, which depend primarily on sleeve gastrectomy as a restrictive element. Recent endoscopic procedures will also be highlighted.
3.2 Surgical Procedures
3.2.1 Sleeve Gastrectomy
The worldwide map has been showing a tremendous tendency towards more performance of SG. Few years ago, it was just regarded as the newest member in the family of bariatric surgical interventions. But recent reports anticipate that SG could be the leading bariatric surgical intervention in the near future (Buchwald and Oien 2009, 2013).
In 2003, this procedure was not actually reported as a standalone surgical intervention. In 2008, more than 4,000 SG’s were performed, still far below the performance rates of other standard procedures, namely RYGB (26,000, 39 %) and gastric banding (28,000, 43 %) (Buchwald and Oien 2009). This was however a clue for a tendency of growth. In 2011, more than 31,000 (27 %) SG were performed, exceeding gastric banding (20,000, 17 %) but still below RYGB (49,000, 43 %) (Buchwald and Oien 2013). This represented however a more than fivefold growth rate of this recent procedure, compared to only 88 % growth of RYGB. In Europe, again this fivefold growth rate of SG was reported between 2008 and 2011, compared to only doubling of RYGB. In the same interval, a 40 % drop in gastric bands was documented (Buchwald and Oien 2013).
Another study analyzed the trend of bariatric surgical intervention in USA from the last quarter of 2008 through the third quarter of 2012 (Nguyen et al. 2013). Data showed a steep increase in the performance rate of SG from 0.9 to 36.3 %, with a concomitant decrease in the use of laparoscopic RYGB from 66.8 to 56.4 %, and of laparoscopic gastric bands from 23.8 to 4.1 %. This was associated with an increase in the number of institutions performing laparoscopic sleeve gastrectomy. The number of those performing laparoscopic RYGB and gastric bands remained stationary throughout the period (Nguyen et al. 2013). Analysis of these data leaves few doubts that SG could be the future leading procedure of bariatric intervention.
Buchwald and his colleagues proposed a possible explanation for this phenomenon (Buchwald and Oien 2013). Every procedure goes through an ascending, then stable or maturity phase, and passes to an aging phase, where new procedures start to replace it to avoid the encountered long-term complications or the unsatisfactory outcome attributed to such a traditional procedure. This applies to gastric banding in Europe, where the oldest bands were placed, and to RYGB in USA where they were first practiced. This decline contrasts with the flourishing of SG, which is currently accepted by the ASMBS as a standalone bariatric surgical intervention (ASMBS 2012).
Compared to RYGB, SG seems to be a relatively easier and quicker procedure. Moreover, it can be practiced in super–super obese patients, in whom RYGB would require greater technical and surgical command. SG also showed very reasonable results, in terms of its weight loss outcome. Data from the fourth international consensus summit on sleeve gastrectomy reported excess weight loss (%EWL) from 50 to 60 %, up to 6 years after SG (Gagner et al. 2013).
SG proved to have significant antidiabetic efficacy, which ranged from 60 to 90 % in recent reports (Rao and Kini 2012). This efficacy was almost comparable to that encountered after the gold standard RYGB at 1 and 2 years postoperatively (de Gordejuela et al. 2011). Proper patient selection assures better postoperative diabetes improvement or remission. Patients with a shorter history of diabetes, those with a less severe diabetic state, and those who encounter better weight loss are candidates for a better antidiabetic effect. Although long-term results are still lacking, 3 years follow-up studies showed an up to 80 % diabetes free status (Abbatini et al. 2010). A recent nationwide survey in Germany reported a 6-year antidiabetic efficacy of SG of 59 %, which was lower than the standard RYGB (83 %), but still promising (Weiner et al. 2014).
Another factor is possibly the lower cost of SG, compared to RYGB. Nguyen and his colleagues reported lower hospital costs (USD $13.081 ± 4.471 vs $14.401 ± 3.851, respectively) and shorter length of stay (2.07 ± 0.92 vs 2.26 ± 1.04 days, respectively) (Nguyen et al. 2013).
A large study from the USA reported that rates of serious complications after SG, although higher than those encountered after gastric banding (2.2 % versus 0.9 %), were lower than after RYGB (3.6 %). Additionally, no mortality was encountered, versus 0.04 % after gastric banding and 0.14 % after RYGB (Birkmeyer et al. 2010). Reports from the American College of Surgeons (Hutter et al. 2011) stated that 30-day morbidity rates after SG are comparable to those after RYGB (5.61 vs. 5.91 %, respectively). Similarly, 30-day readmission rates were comparable in both procedures (5.40 vs 6.47 %, respectively).
In our opinion, another point which played a role in widespread performance of sleeve gastrectomy is the lack of standard second-step procedures in patients with non-satisfactory outcome after RYGB. The available few options after failed RYGB include for example banded RYGB (with subsequent foreign-body-attributed side effects) or distalization (with severe malabsorptive outcome, if patients are not properly monitored). On the other hand, sleeve gastrectomy can be considered as a first-step procedure. If not followed by the expected antiobesity or antidiabetic outcome, it can be converted into malabsorptive second-step procedures, which have a significant additional outcome. Those include for example single anastomotic (mini) gastric bypass, SADI-S (single anastomotic duodeno-ileal bypass sleeve) and the classical BPD-DS. Mini gastric bypass and SADI-S are expected, in our opinion, to have a leading role—either as primary or revisional procedures—in the next few years.
3.2.2 Mini Gastric Bypass
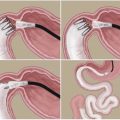
Analysis of worldwide bariatric practice in 2008 didn’t mention MGB (Buchwald and Oien 2009). In 2011, more than 5,000 MGBs were performed worldwide, accounting for about 1.5 % of bariatric practice, which was almost the same as the classical BPD-DS, both coming directly after SG (Buchwald and Oien 2009). We expect flourishing of MGB in the next few years. MGB is a relatively safer and easier alternative, entailing a single anastomosis, with a shorter operative time and lower complication rate compared to the gold standard RYGB (Lee et al. 2012; Peraglie 2008). It omits also the possibility of developing postoperative internal herniation (Fig. 3.1).
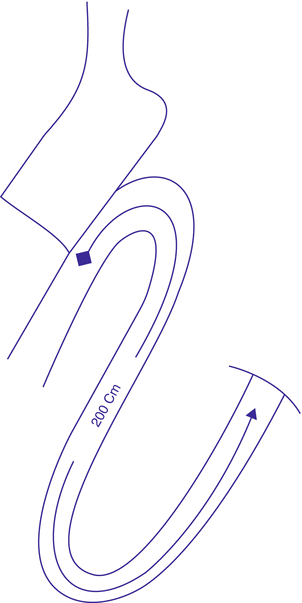

Fig. 3.1
Mini-gastric bypass
Despite its relatively recent nature, this procedure achieved very satisfactory weight loss results for up to 5 years. At 1 year postoperatively, %EWL ranged from 57 % in small-sized studies up to 86 % in larger ones (Mahawar et al. 2013). Results of 80 % EWL at 18 months were reported. At 2 years postoperatively, %EWL ranged from 64 to 92 % in large series. Promising long-term results were reported 5 years postoperatively, EWL exceeding 70 % (Lee et al. 2008). Noun et al. (2007), reported more or less similar results of EWL, reaching 68 % when they followed the patients for up to 5 years.
MGB is in our opinion a promising revisional procedure after a failed sleeve. We analyzed our results in revision, due to insufficient weight loss or comorbidity improvement after sleeve gastrectomy (Weiner et al. 2011). MGB achieved a BMI loss which was significantly higher than that achieved after re-sleeve, RYGB, and banded sleeve as possible alternatives for insufficient weight loss after initial sleeve. The BMI drop experienced with MGB was lower than that encountered with BPD-DS as a possible revisional alternative, but the feasibility of MGB should be put in consideration. In their series of 24 patients, Chakhtoura et al. (2008) converted patients with prior LAGB and vertical gastroplasty using MGB. Rutledge (2006) reported an EWL of 79 % at 1 year follow-up after converting patients with history of LAGB in MGB. Wang et al. (2004), in a series of 29 patients, reported promising results of converting patients with vertical gastroplasty in MGB.
The reported antidiabetic efficacy of a relatively simple procedure like MGB is another supporting factor for its further worldwide spread in the next years. 87 % of patients in the series reported by Lee et al. (2008), experienced successful treatment of their diabetic state. His results were more promising in patients with BMI of more than 35 kg/m2, who showed a noticeable reduction of their glycosylated hemoglobin levels within 1 year after surgery. A diabetes resolution rate of 90 % was reported by Piazza et al. (2011). Kim and Hur (2011), reported a diabetic resolution rate of 70 % in a series of ten patients. Recent reports demonstrated a higher tendency for diabetes remission with MGB, relative to SG (Milone et al. 2013).
3.2.3 Other Recent Bariatric Procedures Involving SG as a Restrictive Element
Several studies discussed the combination of SG (as a restrictive element), with malabsorptive elements, to augment its metabolic and/or weight loss effects. These are all recent procedures with promising results. However, several critical questions are still in need to be thoroughly answered before accrediting these procedures as weight loss/metabolic procedures, and applying them on a large scale. For example, they still lack long-term results. Second, can these procedures be implemented as a second step, in patients who don’t achieve satisfactory results with SG alone? Third, comparative studies between these procedures are currently lacking, namely with BPD/DS and with each other, regarding their metabolic/bariatric outcome.
3.2.4 Single Anastomosis Duodeno-Ileal Bypass with Sleeve Gastrectomy
In 2010, Sánchez-Pernaute et al. published the 3-year postoperative results of their innovative SADI-S technique (Fig. 3.2). This operation entails transection of the first part of the duodenum after construction of a sleeve-like stomach. The transected duodenum is then anastomosed to an ileal loop, with the anastomosis lying 200 cm proximal to the ileocecal junction.
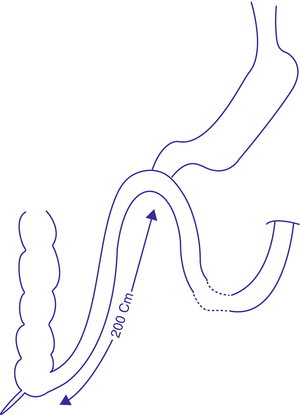

Fig. 3.2
Single anastomosis duodeno-ileal bypass with sleeve gastrectomy (SADI-S)
It has the advantages of the classical BPD/DS, but avoids the risks associated with creating several anastomoses and mesenteric windows. Additionally, the operative time will be shorter. Another advantage here is that the preserved pyloric sphincter will relieve the possibility of gastric pouch cancer development associated with biliary reflux, which is encountered in MGB. This preserved pylorus will also be highly prophylactic against any dumping symptoms, associated with either classical RYGB or MGB. Compared to the classical RYGB, it will have a greater malabsorptive component, with resultant weight loss and comorbidity control. The published results showed excellent weight reduction results. Three months postoperatively, mean %EWL reached 53 %. At 6 months, it exceeded 81 % and reached 87 % at 9 months. %EWL at 1 year reached 94 % and continued to increase to reach 98 % at 18 months and 114 % at 2 years, a percentage which was maintained through the third year.
Among the 50 patients, there were 27 patients with diabetes mellitus (DM) type 2. Mean blood glucose levels dropped from a preoperative value of 174 mg/dl to 97 mg/dl. All diabetic patients stopped their antidiabetic treatment within 6 months after the operation. Resolution of other comorbidities was also achieved.
In 2014, Lee et al. published results of 50 morbidly obese patients who underwent a similar procedure called single-anastomosis duodenal–jejunal bypass with Sleeve Gastrectomy (SADJB-SG). They referred to it also as a short or mini-duodenal switch (Fig. 3.3). It entails a duodeno-jenunal rather than the duodeno-ileal bypass performed by Sánchez-Pernaute et al. Sleeve gastrectomy was performed using a 45Fr bougie, starting 6 cm proximal to the pylorus. A single loop anastomosis was however performed at a distance of 150–200 cm from the ligament of Treitz, to a transected sleeve stomach, at the level of the proximal duodenum (unlike Sánchez who constructed his anastomosis 200 cm proximal to the ileocecal junction). They compared 1-year results prospectively, with classical RYGB (with a 100 cm long biliopancreatic limb and a 150 cm long alimentary limb) and with MGB (a single loop anastomosis to a longer proximal gastric pouch, 150–200 cm distal to the ligament of Treitz).
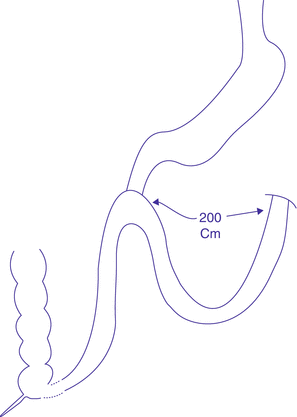

Fig. 3.3
Single-anastomosis duodenal–jejunal bypass with sleeve gastrectomy (SADJB-SG)
In this report, operative time and hospital stay were significantly longer than RYGB and MGB. This may be due to practicing a new procedure, compared to a much higher experience in RYGB and MGB. However, %EWL was significantly higher, compared to RYGB and MGB (80.3 %, 63.4 %, and 68.6 % respectively). Serum high-density lipoprotein level was also significantly higher after SADJB-SG, compared to RYGB and MGB (53.6 mg/dl, 38.6 mg/dl, and 47.1 mg/dl respectively). Unexpectedly, serum cholesterol level was significantly higher after SADJB-SG, compared to RYGB and MGB (188.1 mg/dl, 151.5 mg/dl, and 173.8 mg/dl respectively). Similarly, low-density lipoprotein level was significantly higher after SADJB-SG, compared to RYGB and MGB (114.7 mg/dl, 73.1 mg/dl, and 111.9 mg/dl respectively). They didn’t have a reasonable explanation for this finding. On the other hand, mean glycosylated hemoglobin (HbA1c) level one year after surgery dropped from 9.2 % before surgery to 6.1 % one year after surgery, and 64 % of the diabetic patients showed complete remission.
In the same year, Mui et al. (2014) published 1-year follow-up results of a case report for a novel technique, involving a loop gastroileostomy, with the anastomosis constructed at a distance of 250 cm from the ileocecal junction (Fig. 3.4). They claim that tailoring of this anastomosis will reduce the sleeve tube pressure, which plays a central role in post sleeve gastrectomy leakage. Moreover, gastrografin imaging showed that the preferential contrast passage through the anastomosis obviates the need for duodenal transection. This patient was diabetic, with a BMI of 33 kg/m2 and HbA1c of 10.1 %. At 1 year postoperatively, his BMI dropped to 23.3 kg/m2 and his HbA1c dropped to 4.8 %, with complete withdrawal of antidiabetic therapy 2 months after the operation. This patient developed however mild hypoalbuminemia and anemia, compared to baseline. This might be explained by the preferential passage of food material through the anastomosis, with resultant bypass of intestinal segments.
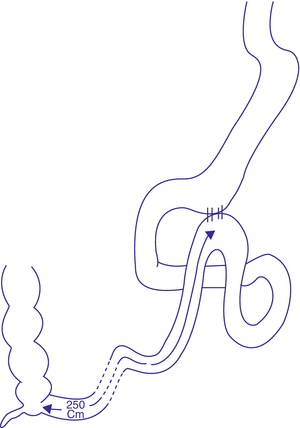

Fig. 3.4
Sleeve gastrectomy with loop bipartition
3.2.5 Sleeve Gastrectomy with Roux-en-Y Duodeno-jejunal Bypass
Several studies in the literature assessed the effect of constructing a sleeve stomach and anastomosing it in a Roux fashion to an intestinal alimentary limb, aiming at achieving a duodeno-jejunal bypass (Tables 3.1 and 3.2, Fig. 3.5). A combined mechanism of action is achieved in this way: the bypass of small intestinal segments contributes to the foregut–hindgut hypothesis formerly described by Rubino (Rubino et al. 2006; Cummings et al. 2007). The constructed sleeve adds a restrictive element together with the reduction in orixigenic ghrelin hormone levels (Langer et al. 2005; Karamanakos et al. 2008).
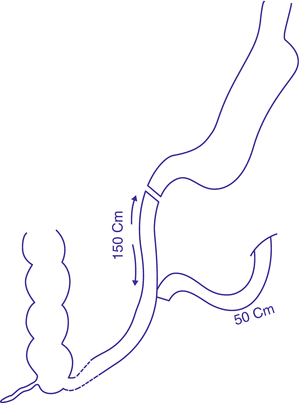
Table 3.1
Results of Roux-en-Y duodeno-jejunal/ileal bypass procedures combined with sleeve gastrectomy
Author and year | Mean operative duration (min) | Number of patients | Bougie size (Fr) | Length of BPL (cm) | Length of alimentary limb (cm) | Length of CC (cm) | Complications: Number (%) | %EWL (follow-up duration in months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3 | 6 | 9 | 12 | 18 | 24 | 36 | 48 | 60 | |||||||||
Sleeve gastrectomy with transit bipartition | Santoro et al. (2012) | 170 | 1,020 | 36 | – | 180 | 80 | • Fistula: 9 (0.9) • Bleeding: 8 (0.8 %) • Reoperation: 19 (1.9 %) | 46.3 | 72.2 | 91 | 94 | 85 | 78 | 74 | ||
Sleeve gastrectomy with Roux-en-Y duodeno-jejunal bypass | Raj et al. (2012) | 143 | 38 | – | – | – | Reoperation due to internal herniation in 1 patient | 34 | 60 | 71 | 73 | ||||||
Navarrete et al. (2011) | 148 | 10 | 60 | 50 | 100 | – | • Transfusion due to intra-abdominal bleeding in 1 patient • Surgical wound infection in 1 patient | Average weight loss = 8.5 kg | |||||||||
Kasama et al. (2009) | 217 | 21 | 45 | 50–100 | 150–200 | – | Reoperation due to leak at angle of His in 1 patient | 47 | 63 | 66 | 78 | 96 | |||||
Table 3.2
Results of Roux-en-Y duodeno-jejunal/ileal bypass procedures combined with sleeve gastrectomy
Author and year | Effect on diabetes mellitus | Effect on hyperlipidemia | Effect on hypertension | Effect on respiratory problems | Effect on orthopedic complains | Follow-up duration | |
|---|---|---|---|---|---|---|---|
Sleeve gastrectomy with transit bipartition | Santoro et al. (2012) | • 86 % complete remission • 14 % improvement | • Hypertriglyceridemia improved in 85 % of patients • Hypercholesterolemia improved in 70 % of patients | Resolution in 72 % of patients | • 91 % resolution • 9 % improvement | • 83 % resolution • 17 % improvement | 5 years in 59.1 % of patients |
Sleeve gastrectomy with Roux-en-Y duodeno-jejunal bypass | Raj et al. (2012) | • Remission in 19 patients (73 %) • Improvement in 5 patients (19 %) • Dropped level of HbA1c in 2 patients (8 %) | • Resolution in 19 patients (86 %) • Improvement in 3 patients (14 %) | • Resolution in 11 patients (69 %) • Improvement in 3 patients (19 %) • No effect in 2 patients (12 %) | Mean follow-up period of 17 months | ||
Navarrete et al. (2011) | • Remission in 4 patients (40 %) • Improvement in 3 patients (30 %) • Control in 3 patients (30 %) | Serum cholesterol and triglyceride levels are normalized in all patients | 12 months | ||||
Kasama et al. (2009) | • 93 % resolution (13 patients) • 7 % improvement (1 patient) | Resolution in 11 patients (100 %) | • Resolution in 7 patients (86 %) • Improvement in 1 patient (14 %) | 18 months |

Fig. 3.5
Sleeve gastrectomy with Roux-en-Y duodeno-jejunal bypass
3.2.6 Sleeve Gastrectomy with Transit Bipartition
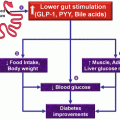
In 2012, Santoro et al. published results of transit bipartition in more than 1,000 patients. This large-sized series discussed the technique and outcome of a novel procedure, based on anastomosing a Roux-en-Y ileal loop to the lowest part of the constructed sleeve without duodenal dissection or division (Fig. 3.6). It is a simplified modification of BPD/DS omitting the step of duodenal division. This has several advantages. First, duodeno-jejunal bypass avoids excessive absorption of fat rich diet in obese patient, which prove to be exaggerated in the first 70 cm of the small bowel. Second, ileal shift of nutrients induces enterohormonal changes with resultant stimulation of secretion of glucagon-like peptide 1 (GLP-1), which is a fasting state-inducing distal bowel hormone, with significant antidiabetic effect (Santoro et al. 2012; De Paula et al. 2010).
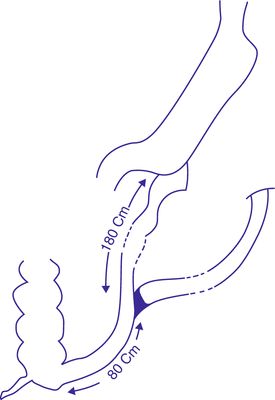

Fig. 3.6
Sleeve gastrectomy with transit bipartition
At the same time, maintaining the passage of nutrients through the duodeno-jejunal tract, although much lower than in normal, will avoid the undesirable malnutritional outcome encountered in BPD/DS, mainly the hypoalbuminemic state. Maintaining an endoscopic access to the duodeno-jejunal segment is another privilege of this procedure over the classical BPD/DS (Santoro et al. 2012). Tables 3.1 and 3.2 summarizes the results of some published Roux-en-Y duodeno-jejunal/ileal bypass procedures combined with sleeve gastrectomy.
3.2.7 Ileal Interposition
De Paula and other authors published several series, since the middle of the last decade till recently (DePaula et al. 2008, 2012; Kota et al. 2012; Goel et al. 2011; Tinoco et al. 2011; Kumar et al. 2009). They discussed the outcome of a novel technique including a combination of ileal interposisition and sleeve gastrectomy. Ileal interposition focuses on inducing a neuroendocrine brake, aiming at ameliorating the effects of metabolic syndrome, apart from its weight reduction effects. This technique included two different modalities: the duodenal ileal interposition-sleeve gastrectomy, DII-SG (Fig. 3.7), and the jejunal–ileal interposition-sleeve gastrectomy, JII-SG (Fig. 3.8, Tables 3.3, 3.4, 3.5, and 3.6) (DePaula et al. 2008, 2012).
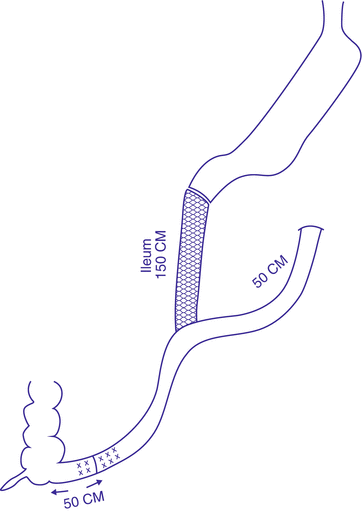
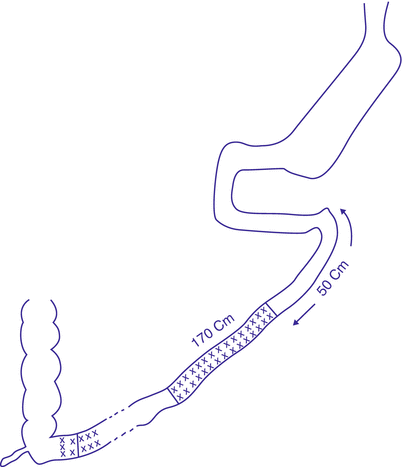

Fig. 3.7
Duodenal ileal interposition-sleeve gastrectomy, DII-SG

Fig. 3.8
Jejunal–ileal interposition-sleeve gastrectomy, JII-SG
Table 3.3
Results of studies addressing ileal interposition
Author and Year | Number of patients | Sleeve bougie size (French) | Type of operation | Preoperative BMI (kg/m2) mean ± SD (Range) | Age Mean ± SD (Range) | Gender m/f | Follow-up in months mean ± SD (Range) | Complications | Mortality | Mean postoperative BMI (±SD) (kg/m2) |
|---|---|---|---|---|---|---|---|---|---|---|
De Paula et al. (2010) | 202 | 38 | • JII-SG (125 patients) • DII-SG (77 patients) | 29.7 ± 3.5 (21.5–34.9) | 52.2 ± 7.5 (29–72) | 70.8 % / 29.2 % | 39 ± 9 (25–61) | 1 % leak rate | • Early:1 % • Late:1 % | 23.5 ± 3.1a |
Kota et al. (2012) | 17 | 32–60 | DII-SG | 29.2 ± 7.5 (22.4–37.5) | 50.7 ± 8.1 (34–66) | 12/5 | 9.1 ± 5.3 (3–21) | Ileal perforation (1 patient) | None | 22.1 ± 2.9a after 1 year |
Goel et al. (2011) | 5 | 36 | JII-SG | 29.4 | 47.33 | 2/3 | 6 | No major complications | None | Mean BMI drop was 8.4 kg/m2 |
Tinoco et al. (2011) | 30 | 32 | JII-SG | 30.8 ± 5.1 | 49.7 ± 8.9 | 20/10 | 13 ± 3.3 (6-18) | • Metabolic ketoacidosis (1 patient) • Urinary tract infection (1 patient) • Diarrhea (2 patients) | None | 25.7 ± 4a |
Kumar et al. (2009) | 10 | 32–58 | JII-SG | 33.8 ± 6.5 (25.5-45.5) | 48.2 ± 9 (34-62) | 4/6 | 2-16 | No major complications | None | 26.2 |
Table 3.4




Results of studies addressing ileal interposition
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree