Highlights
- •
A study in patients with unresectable or metastatic urothelial carcinoma (mUC).
- •
Half of patients received cisplatin-based regimens as first-line therapy.
- •
After 2017, 58.2% of patients received pembrolizumab as second-line therapy.
- •
After 2017, 19.1% of patients received enfortumab vedotin as third-line therapy.
- •
Overall survival of patients with mUC remains poor.
Abstract
Background
Cisplatin-based chemotherapy has traditionally been the standard treatment for unresectable or metastatic urothelial carcinoma (mUC). Recently, the longstanding paradigm has changed with the emergence of immune checkpoint inhibitors and antibody-drug conjugates, such as pembrolizumab and enfortumab vedotin (EV). This longitudinal descriptive study aimed to identify real-world treatment patterns and assess the outcomes of patients with mUC between 2010 and 2023.
Methods
Patients with mUC who received first-line systemic therapy were identified from a Japanese electronic medical records database. A Sankey diagram was used to present the proportion of patients who transitioned to second- and third-line therapies. Kaplan–Meier survival analysis was used to estimate the time to next treatment (TTNT) and overall survival (OS).
Results
A total of 794 patients were included in this study. The median age of the patients was 73.0 years, and 72.9% were male. The most common primary tumor site was the bladder (59.7%). First-line therapy comprised cisplatin-based regimens in 52.0% of the patients (11.8% at standard doses, 32.4% at reduced doses, and 7.8% at unknown doses), carboplatin-based regimens in 32.1%, and other regimens in 15.9%. Among the patients enrolled after 2017, following the approval of pembrolizumab for mUC progressing after chemotherapy in Japan, 58.2% received pembrolizumab as second-line therapy, and 19.1% received EV monotherapy as third-line therapy. The median OS for the total population was 24.1 months, with patients enrolled between 2010 and 2016 having a shorter OS (21.1 months) than those enrolled between 2017 and 2022 (24.9 months). For patients with eGFRs of ≥60 and <60 mL/min/1.73 m 2 , the median OS was 24.1 and 23.8 months, respectively.
Conclusion
Platinum-based regimens, including reduced-dose cisplatin and carboplatin, remain the predominant first-line systemic therapies. Since 2017, pembrolizumab and EV have become widespread choices for second-line and subsequent treatments, gradually surpassing the previously prevalent platinum-based regimens. The introduction of these novel therapies might have prolonged the OS of patients with mUC. A plain language summary is available in this article.
1
Introduction
Since the 1990s, cisplatin-based chemotherapy has been the standard first-line treatment for unresectable or metastatic urothelial carcinoma (mUC) [ ]. However, many patients are ineligible due to poor performance status or impaired renal function [ ]. Consequently, carboplatin- and taxane-based chemotherapies emerged as alternatives [ ]. Disease progression after first-line treatment highlights the need for effective second-line therapies.
Recent years have witnessed a paradigm shift in mUC treatment with the introduction of immune checkpoint inhibitors (ICIs) targeting programmed death receptor-1 (PD-1) or programmed death ligand-1 (PD-L1) [ ]. By 2023, several ICIs had been approved in the US and Europe for platinum-ineligible patients with mUC and those progressing after platinum-based chemotherapy [ ]. However, only pembrolizumab has been approved in Japan for the treatment of mUC progressing after chemotherapy. Avelumab is indicated in the US, Europe, and Japan for maintenance therapy in patients without disease progression on platinum-based chemotherapy.
Another significant development is enfortumab vedotin (EV), an antibody-drug conjugate (ADC) targeting Nectin-4. EV has been proven to prolong survival in patients with mUC after platinum chemotherapy and ICIs, even those ineligible for cisplatin [ ]. In the US, EV combined with pembrolizumab has received approval as a first-line option for cisplatin-ineligible mUC. The efficacy of this combination was confirmed in a phase III trial involving platinum-eligible patients [ ]. As of April 2024, this combination regimen is awaiting approval as a first-line option in both in Europe and Japan, although EV monotherapy has already been approved as a third-line treatment option.
The evolving mUC treatment landscape emphasizes the importance of understanding real-world treatment patterns and outcomes [ ]. While studies in the US and Europe have elucidated treatment strategies and survival [ ], there remains a significant knowledge gap regarding treatment patterns and outcomes in Japan, particularly concerning the use of ICIs and EV. Thus, we analyzed the characteristics, treatment patterns, and outcomes of patients with mUC over the past decade using a Japanese electronic medical records (EMRs) database.
2
Materials and methods
2.1
Study design and data source
This longitudinal descriptive study utilized the RWD database managed by the Health, Clinic, and Education Information Evaluation Institute (HCEI) with assistance from Real World Data Co., Ltd. [ , ]. As of 2023, the database included EMRs, claims data, and diagnosis procedure combination (DPC) data for approximately 14.1 million patients treated in 190 medical institutions throughout Japan. This database contains information on demographics, inpatient and outpatient diagnoses, procedures, medications, and laboratory test results. Patient-level data can be tracked within the same medical institution. Our dataset included data collected between January 27, 1985, and May 20, 2023. This study was approved by the nonprofit MINS Research Ethics Committee (approval number: 230207). Due to the anonymized retrospective nature of the study, the requirement for informed consent was waived. An opt-out approach was used to obtain patient consent from each medical institution.
2.2
Study population
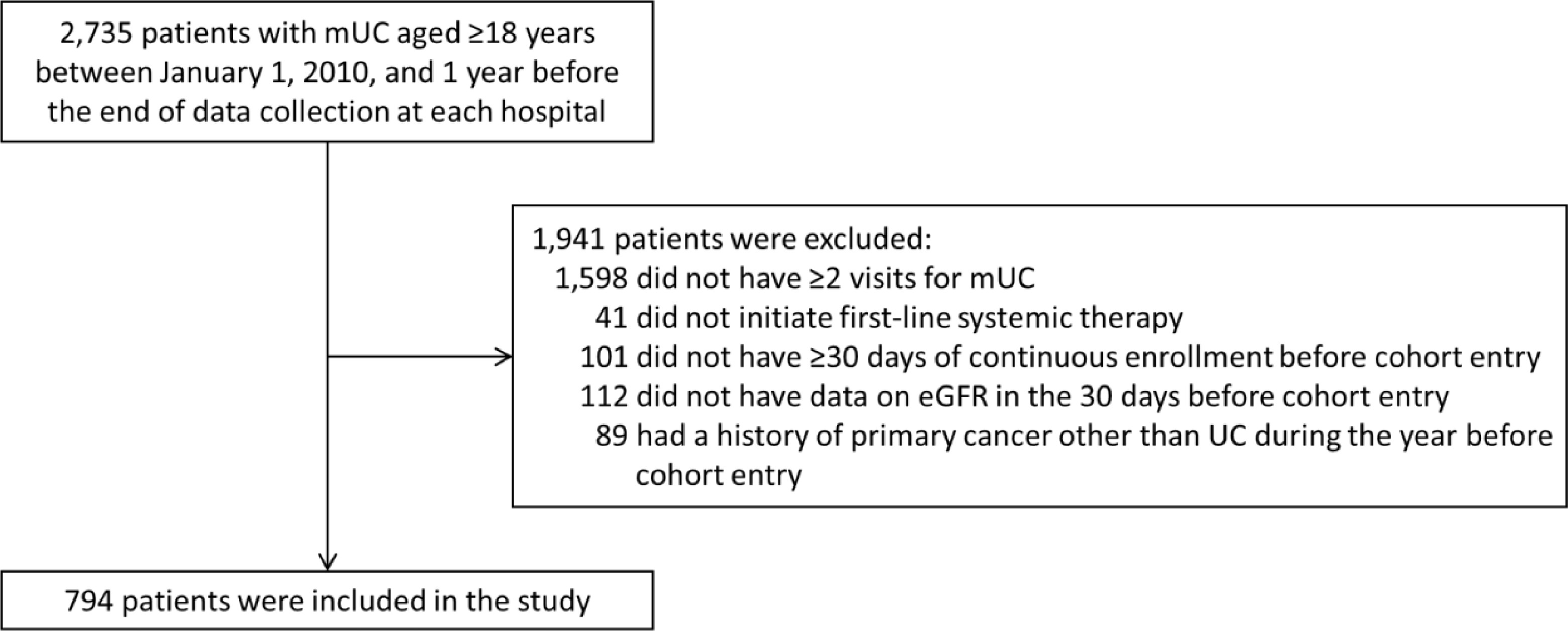
We identified patients aged ≥18 years with mUCs. To ensure diagnostic validity, we included only patients with ≥2 visits for mUC at least 30 days apart. Patients were required to have initiated one of the following regimens as the first-line therapy for mUC: (1) cisplatin-based, (2) carboplatin-based, or (3) other regimens. The start date of first-line therapy was defined as the cohort entry date and was required to be between January 1, 2010, and 1 year before the end of data collection at each medical institution. Additionally, they were required to have been continuously enrolled in the database for ≥30 days before cohort entry and to have had data on estimated glomerular filtration rate (eGFR) within the previous 30 days [ ]. Patients were excluded if they had a primary cancer other than urothelial carcinoma (UC) in the year before cohort entry. Supplementary Table 1 outlines the eligibility.
2.3
Study outcomes and follow-up
The primary outcome comprised treatment patterns, including treatment regimens and sequences. Treatment regimens included (1) cisplatin-based regimens (e.g., gemcitabine and cisplatin [GC], methotrexate, vinblastine, doxorubicin, and cisplatin [standard MVAC], and dose-dense MVAC [ddMVAC]); (2) carboplatin-based regimens (e.g., gemcitabine and carboplatin [GCarbo] and carboplatin and paclitaxel [CP]); and (3) other regimens (e.g., taxane-based chemotherapies, pembrolizumab, avelumab, and EV) ( Supplementary Table 2 ). The treatment sequence was evaluated from first- to third-line therapy.
The secondary outcomes comprised time to next treatment (TTNT) and overall survival (OS). Due to the unavailability of progression-free survival data in our dataset, TTNT was selected as a surrogate outcome. TTNT was calculated from the initiation of first-line therapy to the commencement of subsequent therapy or death and OS from the initiation of first-line therapy to death, both censored at the last visit.
2.4
Patient and institutional characteristics
The patient and institutional characteristics at baseline included (1) demographics, (2) cohort entry year, (3) primary site of UC, (4) de novo advanced or recurrent UC, (5) perioperative chemotherapy, (6) comorbidities, (7) antiresorptive therapy for bone metastases, (8) eGFR, and (9) hospital size by number of beds. Definitions are provided in Supplementary Table 3 .
2.5
Statistical analysis
Patient characteristics were summarized using descriptive statistics both in the total population and in groups stratified by first-line therapy (cisplatin-based, carboplatin-based, and other regimens). When classifying cisplatin-based regimens into standard and reduced doses, if a patient’s body surface area data were missing, the cisplatin regimen was categorized as having an unknown dose. A Sankey diagram was used to present the proportion of patients who transitioned to second- and third-line therapies. Kaplan–Meier survival analysis was used to estimate TTNT and OS, stratified by first-line regimen.
We also conducted subgroup analyses stratified by (1) cohort entry year (2010–2016, 2017–2022; 2017 is the year pembrolizumab was approved for mUC progressing after chemotherapy in Japan); (2) eGFR categorized into (i) <30, 30–<45, 45–<60, ≥60 mL/min/1.73 m 2 and (ii) <60, ≥60 mL/min/1.73 m 2 ; (3) age (<70, 70–<80, ≥80 years; this analysis was exclusively conducted for examining treatment patterns); and (4) primary site of UC (upper urinary tract [renal pelvis, ureter], lower urinary tract [bladder, urethra]). Sankey diagrams were generated using R version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria), and other analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC, USA).
3
Results
3.1
Patient characteristics
In total, 794 patients with mUC were eligible for this study ( Fig. 1 ). The median age was 73.0 years (interquartile range [IQR], 68.0–78.0), and 72.9% were male ( Table 1 ). The bladder was the most common primary site (59.7%), followed by the upper urinary tract (39.5%), and urethra (0.8%). The 2010–2016 and 2017–2022 cohorts comprised 25.7% and 74.3% of the patients, respectively. The median eGFR was 49.6 mL/min/1.73 m 2 (IQR, 39.0–65.2): eGFR of <30 in 8.1% of patients; 30–<45, 29.2%; 45–<60, 31.4%; and ≥60, 31.4%.
| Characteristic | Total population | Cisplatin-based regimens | Carboplatin-based regimens | Other regimens |
|---|---|---|---|---|
| Patients * | 794 (100) | 413 (52.0) | 255 (32.1) | 126 (15.9) |
| Age, median (IQR), years | 73.0 (68.0–78.0) | 73.0 (67.0–78.0) | 75.0 (70.0–80.0) | 72.0 (66.0–78.0) |
| <60 | 55 (6.9) | 38 (9.2) | 7 (2.7) | 10 (7.9) |
| 60–<70 | 199 (25.1) | 112 (27.1) | 54 (21.2) | 33 (26.2) |
| 70–<80 | 387 (48.7) | 198 (47.9) | 128 (50.2) | 61 (48.4) |
| ≥80 | 153 (19.3) | 65 (15.7) | 66 (25.9) | 22 (17.5) |
| Sex | ||||
| Male | 579 (72.9) | 303 (73.4) | 177 (69.4) | 99 (78.6) |
| Female | 215 (27.1) | 110 (26.6) | 78 (30.6) | 27 (21.4) |
| Cohort entry year | ||||
| 2010–2016 | 204 (25.7) | 130 (31.5) | 63 (24.7) | 11 (8.7) |
| 2017–2022 | 590 (74.3) | 283 (68.5) | 192 (75.3) | 115 (91.3) |
| Primary site of UC | ||||
| Bladder | 474 (59.7) | 269 (65.1) | 132 (51.8) | 73 (57.9) |
| Renal pelvis | 145 (18.3) | 78 (18.9) | 43 (16.9) | 24 (19.0) |
| Ureter | 169 (21.3) | 62 (15.0) | 79 (31.0) | 28 (22.2) |
| Urethra | 6 (0.8) | 4 (1.0) | 1 (0.4) | 1 (0.8) |
| De novo advanced or recurrent UC | ||||
| De novo advanced | 424 (53.4) | 239 (57.9) | 128 (50.2) | 57 (45.2) |
| Recurrence | 370 (46.6) | 174 (42.1) | 127 (49.8) | 69 (54.8) |
| Perioperative chemotherapy | ||||
| NAC | 96 (12.1) | 39 (9.4) | 16 (6.3) | 41 (32.5) |
| AC | 84 (10.6) | 20 (4.8) | 19 (7.5) | 45 (35.7) |
| Comorbidities | ||||
| Diabetes | 317 (39.9) | 155 (37.5) | 99 (38.8) | 63 (50.0) |
| Hypertension | 355 (44.7) | 167 (40.4) | 127 (49.8) | 61 (48.4) |
| Heart failure | 111 (14.0) | 49 (11.9) | 39 (15.3) | 23 (18.3) |
| COPD | 74 (9.3) | 33 (8.0) | 23 (9.0) | 18 (14.3) |
| Neurological disorders | 89 (11.2) | 47 (11.4) | 18 (7.1) | 24 (19.0) |
| DVT | 34 (4.3) | 17 (4.1) | 8 (3.1) | 9 (7.1) |
| VTE | 37 (4.7) | 18 (4.4) | 8 (3.1) | 11 (8.7) |
| Antiresorptive therapy for bone metastases a | 42 (41.2) | 21 (45.7) | 11 (30.6) | 10 (50.0) |
| eGFR, median (IQR), mL/min/1.73 m 2 | 49.6 (39.0–65.2) | 58.1 (46.5–70.2) | 42.7 (34.2–54.6) | 40.8 (34.9–58.5) |
| <30 | 64 (8.1) | 12 (2.9) | 28 (11.0) | 24 (19.0) |
| 30–<45 | 232 (29.2) | 74 (17.9) | 111 (43.5) | 47 (37.3) |
| 45–<60 | 249 (31.4) | 146 (35.4) | 74 (29.0) | 29 (23.0) |
| ≥60 | 249 (31.4) | 181 (43.8) | 42 (16.5) | 26 (20.6) |
| Hospital size by number of beds | ||||
| 0–<100 | 0 | 0 | 0 | 0 |
| 100–<300 | 68 (8.6) | 34 (8.2) | 23 (9.0) | 11 (8.7) |
| 300–<500 | 324 (40.8) | 194 (47.0) | 87 (34.1) | 43 (34.1) |
| ≥500 | 402 (50.6) | 185 (44.8) | 145 (56.9) | 72 (57.1) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree