Reactive, Inflammatory, and Nonproliferative Lesions
Some reactive, inflammatory, and nonproliferative lesions of the breast present problems clinically but are treated without resorting to a biopsy. In others, a biopsy is required to make the correct diagnosis and to distinguish the process from malignancy. In this chapter, reactive and inflammatory conditions that are most likely to be encountered in breast biopsies are discussed first, followed by brief considerations of other conditions in these categories. Finally, several common nonproliferative lesions are considered.
BIOPSY SITE CHANGES
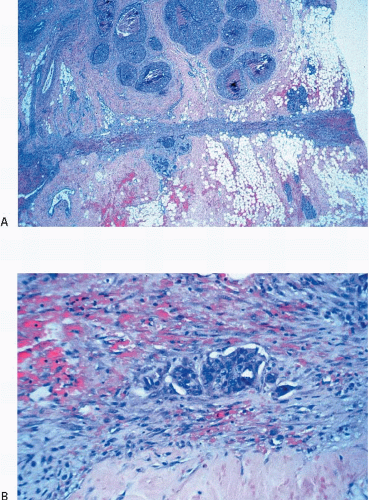
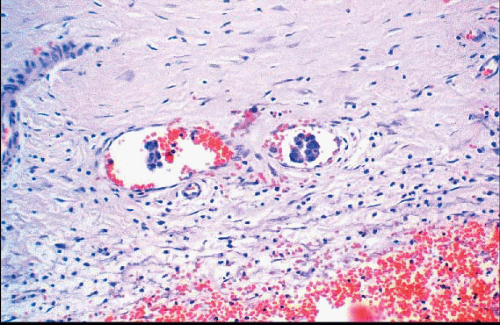
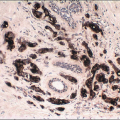
Changes related to a prior biopsy are the most common reactive lesions encountered in the breast. The details of the changes observed are related to a variety of factors including the type of procedure performed (needle vs. open surgical biopsy) and the time interval between the original procedure and removal of the subsequent specimen. Post-biopsy changes in the breast that are similar to those encountered elsewhere include recent and organizing hemorrhage, fat necrosis, acute and chronic inflammation, foreign body giant cell reaction, granulation tissue, and scarring (Fig. 2.1, e-Fig. 2.1).
Reactive spindle cell nodules (RSCNs) similar in appearance to the postoperative spindle cell nodules reported in the genitourinary tract and thyroid may also occur in the breast.1 These are most commonly seen following needle biopsy trauma to lesions with a prominent fibrous stromal component such as papillary lesions and complex sclerosing lesions. RSCNs are composed of intersecting fascicles of plump spindle cells with admixed blood vessels, collagen fibers, and a mixed inflammatory infiltrate including lymphocytes, plasma cells, and macrophages, some of which are hemosiderin-laden. The spindle cells show mild to moderate pleomorphism, rare mitotic figures, and an immunophenotype consistent with myofibroblasts. While distinguishing RSCNs from other spindle cell lesions of the breast may be challenging, a history of a previous needling procedure and the presence of other changes associated with biopsy sites such as hemosiderin and foamy histiocytes favor a diagnosis of RSCN.
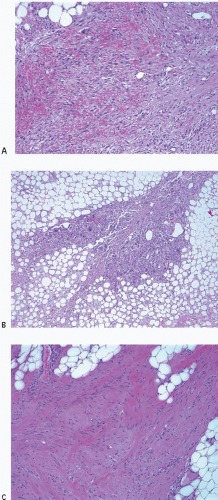 FIGURE 2.1 Biopsy site changes include organizing hemorrhage (A), fat necrosis and foreign body giant cell reaction (B), and scarring (C). |
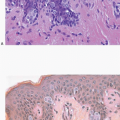
Squamous metaplasia may be seen in duct and lobular epithelium in the vicinity of a prior biopsy site, and in some cases, the metaplastic epithelium completely replaces the native glandular epithelium (Fig. 2.2, e-Fig. 2.2).
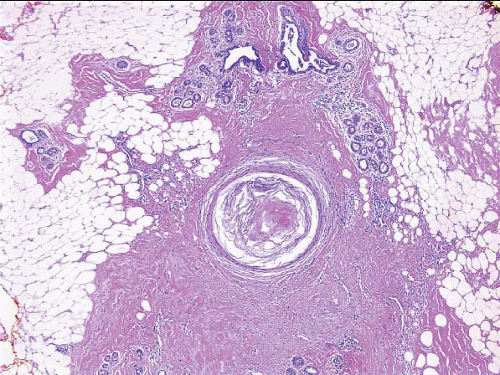
Partial or total infarction of lesions, especially papillary lesions and fibroepithelial lesions, may occur following sampling by fine-needle aspiration or core-needle biopsy (CNB), and this can confound the interpretation of subsequent excision specimens (Fig. 2.3, e-Fig. 2.3). Total infarction can preclude the definitive categorization of a lesion as benign, atypical, or malignant.
There are a number of additional alterations seen following a CNB. Marking devices are typically placed in the breast following the CNB procedure so that the location of the biopsy site can be identified on subsequent radiologic examination. One type is composed of pellets of a resorbable copolymer of polylactic acid/polyglycolic acid (similar to Vicryl suture material); within one of the pellets is a stainless steel marker. The copolymer pellets, which dissolve during tissue processing, are initially associated with a fibrotic rim, with sparse lymphocytes and eosinophils, around empty spaces. Later biopsies show a histiocytic and foreign body-type giant cell reaction around the empty spaces and infiltration of the spaces by fibrinous material. Another type consists of a plug of bovine collagen that contains a titanium clip. The collagen plugs appear as broad bands of eosinophilic, acellular material. There is an accompanying inflammatory infiltrate composed of lymphocytes, plasma cells, eosinophils, and occasionally neutrophils, initially at the periphery of the plug but later within the plug, admixed with the collagen fibers. Foreign body-type giant cells are uncommon (Fig. 2.4, e-Fig. 2.4). With time, the plug is infiltrated by granulation tissue and there is deposition of native collagen.2 A third type of marking device is composed of a biodegradable hydrogel polymer containing a metal marker. After placement in the breast, the device expands by hydration. Subsequent degradation of the polymer material results in cystically dilated spaces surrounded by histiocytes. On low-power examination, these areas can thereby be mistaken for dilated ducts and this can create difficulty in identifying the area as a biopsy site.3
Occasionally, squamous epithelial-lined cysts may be present at the CNB site (Fig. 2.5, e-Fig. 2.5). These may result from displacement of the epidermis or epithelium from cutaneous appendages into the biopsy site or from squamous metaplasia of duct epithelium.4
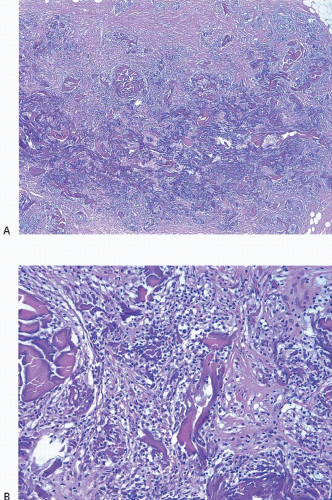
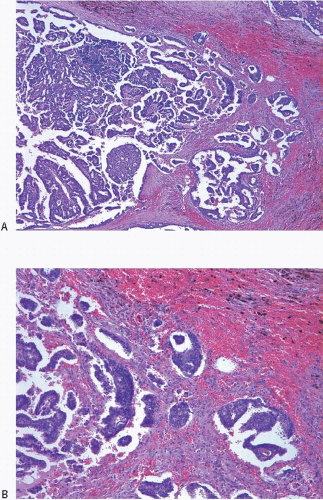
The CNB procedure (and fine-needle aspiration biopsy procedures) may also result in lesion disruption and the displacement of epithelium into the stroma and/or vascular spaces.5, 6, 7 and 8 The likelihood of finding epithelial displacement appears to be inversely related to the length of time between the CNB and subsequent surgical excision. Epithelial displacement into the stroma is particularly common following a CNB of papillary lesions9 but may also be seen following biopsy of other benign lesions and ductal carcinoma in situ (DCIS). Epithelial displacement into axillary lymph nodes may also occur (see Chapter 18).
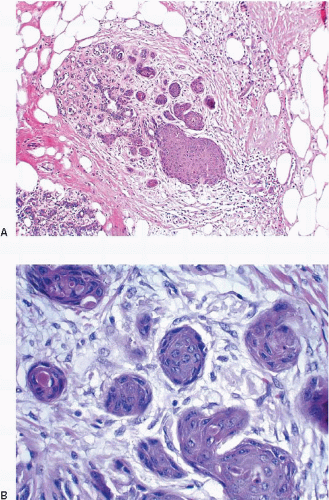
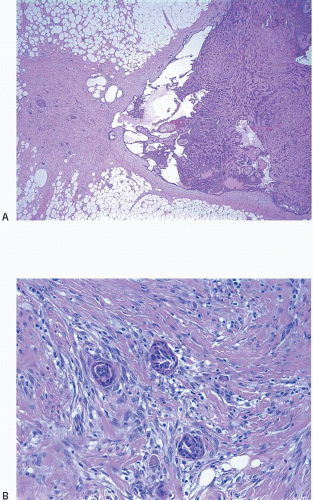
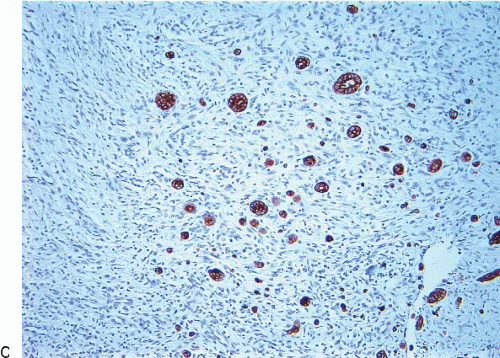
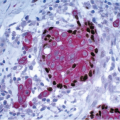
The finding of displaced epithelial cells in the stroma may result in the erroneous diagnosis of invasive carcinoma in patients with benign lesions or DCIS (Figs. 2.6 and 2.7, e-Fig. 2.6) or in the erroneous diagnosis of lymphovascular invasion. In some cases, particularly following biopsy of papillary lesions, the stroma may contain numerous nests of epithelium that show varying degrees of degenerative changes and, not infrequently, squamoid features (Fig. 2.8, e-Fig. 2.7). When epithelial fragments or clusters are confined to the organizing hemorrhage, granulation tissue, or scar of the needle biopsy site, a diagnosis of epithelial displacement should be favored. A diagnosis of invasive carcinoma should be considered only if epithelial cell nests are present in the stroma clearly away from the biopsy site and/or have features characteristic of a recognized type of invasive cancer. This is particularly important in the absence of a prior diagnosis of invasive carcinoma. Immunostains for myoepithelial markers are of value in distinguishing between displaced epithelium and invasive carcinoma only if they demonstrate the presence of myoepithelial cells around the epithelial nests, a feature indicating a benign lesion. However, in many instances, particularly in cases of DCIS and in papillary lesions, the epithelial cells alone are displaced into the stroma; therefore, absence of myoepithelial cells cannot be used as evidence of an invasive process (Fig. 2.9).
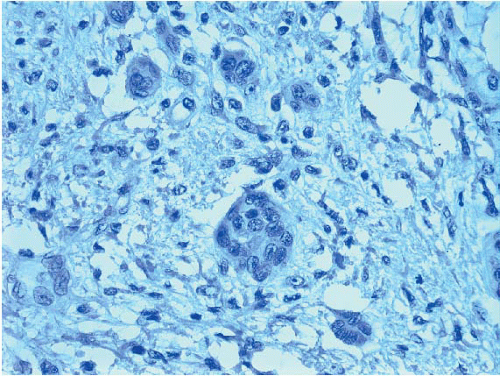 FIGURE 2.9 Immunostain for p63 shows no myoepithelial cells in association with these displaced epithelial cells (same case as in Fig. 2.8). The lack of myoepithelial cells in this setting cannot be used as evidence of invasive carcinoma. |
Epithelial displacement into lymphovascular spaces can be even more problematic (Fig. 2.10). In patients with invasive carcinoma, it may not be possible to distinguish artifactual displacement of epithelial cells from bona fide lymphovascular invasion. In the absence of documented invasive carcinoma, the interpretation of epithelial cells in vascular spaces as lymphovascular invasion by carcinoma should be made with extreme caution, particularly if the involved lymphovascular spaces are confined to the area of the needle biopsy site.
FAT NECROSIS
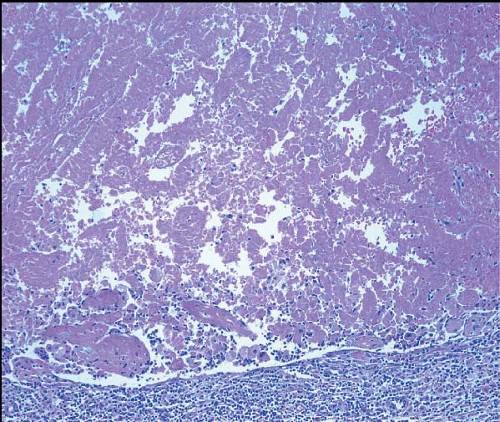
Fat necrosis most commonly occurs following physical injury to the breast (e.g., surgery, needling procedures, radiation, and trauma), but in approximately half of the cases, no history of injury can be elicited. The importance of fat necrosis lies in the fact that it may closely simulate carcinoma both clinically and on mammographic examination.
The macroscopic appearance of fat necrosis depends on its age. In early lesions, there is hemorrhage and indurated fat. With time, a firm mass is formed. The cut surface of the lesion at this stage has a variegated, yellow-gray appearance with focal hemorrhage. Cavitation may subsequently occur as a result of liquefactive necrosis. The lesion may eventually be converted to a dense, fibrous scar or may remain a cystic
cavity with calcification of its walls. The term membranous fat necrosis has been used to describe these cyst-like lesions.10
cavity with calcification of its walls. The term membranous fat necrosis has been used to describe these cyst-like lesions.10
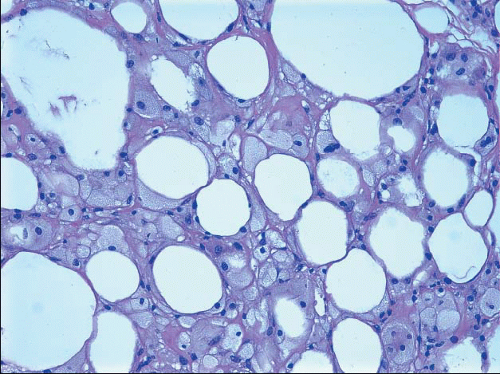
Upon microscopic examination, early lesions show cystic spaces surrounded by lipid-laden histiocytes and foreign body-type giant cells with foamy cytoplasm (Fig. 2.11, e-Fig. 2.8). A variable, acute inflammatory cell infiltrate may be present, and there may be focal hemorrhage. With time, there is fibroblastic proliferation and deposition of collagen. Scattered, chronic inflammatory cells are usually present, and focal hemosiderin deposition may be observed. Even in older lesions, foamy histiocytes and foreign body-type giant cells are usually discernible.
REACTIONS TO FOREIGN MATERIAL
Foreign body-type granulomatous inflammation has been described following injection of a variety of substances, including paraffin and silicone, into the breast. Clinically, these lesions generally appear as firm nodules that may be tender.
A variety of tissue reactions have been reported in association with mammary implants.11 One of these is the formation of a fibrous capsule in the surrounding tissue. In 10% to 40% of patients, there is contracture of this capsule that results in breast tightness or firmness and deformation of the implant, necessitating either capsulotomy or removal of the implant
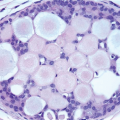
and the surrounding capsule. Histological examination of the capsular tissue shows varying degrees of fibrosis, chronic inflammation, fat necrosis, granulation tissue, fibrin deposition, histiocytes, and foreign body giant cells. Additionally, in the case of silicone gel implants, silicone (and where it has been used as part of the implant shell, polyurethane) may be present within the capsule. Silicone gel leakage may be seen even in the absence of implant rupture and characteristically produces oval, cystic spaces that appear empty or contain amorphous, pale material, which is not birefringent with polarized light (Fig. 2.12, e-Fig. 2.9).12 Silicone can diffuse to a variety of sites around the body, and silicone lymphadenopathy has been reported in axillary lymph nodes (see Chapter 18); hematogenous dissemination can also occur. Implant-associated lymphomas have also been described (see Chapter 14).
and the surrounding capsule. Histological examination of the capsular tissue shows varying degrees of fibrosis, chronic inflammation, fat necrosis, granulation tissue, fibrin deposition, histiocytes, and foreign body giant cells. Additionally, in the case of silicone gel implants, silicone (and where it has been used as part of the implant shell, polyurethane) may be present within the capsule. Silicone gel leakage may be seen even in the absence of implant rupture and characteristically produces oval, cystic spaces that appear empty or contain amorphous, pale material, which is not birefringent with polarized light (Fig. 2.12, e-Fig. 2.9).12 Silicone can diffuse to a variety of sites around the body, and silicone lymphadenopathy has been reported in axillary lymph nodes (see Chapter 18); hematogenous dissemination can also occur. Implant-associated lymphomas have also been described (see Chapter 14).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree