BACKGROUND
The adverse effects of hyperglycemia on the fetus have been recognized since women with diabetes first began to survive and reproduce. Very high rates of late pregnancy fetal loss, and excessive fetal growth were recognized several years ago. In the observational study of Karlsson and Kjellmer, for instance, evidence appeared to suggest that perinatal mortality, if not morbidity, could be mediated by impaired maternal glycemic control.1 The recognition that similar risks of increased perinatal mortality and morbidity were present in gestational diabetes really started with the studies of O’ Sullivan in Boston.
In the late 1950s, O’Sullivan and his colleagues identified a normal range for the 100-g oral glucose tolerance test (OGTT) in pregnancy, which is the basis for screening criteria still used commonly in the US today. In a series of pioneering experiments, they established by randomized controlled trial that untreated GDM was associated with an excess of perinatal mortality and fetal macrosomia. They randomized women with GDM to treatment with prophylactic insulin (20 U of isophane, 10 U of regular insulin) each morning, or no treatment, and compared them with a non-diabetic control group. Insulin treatment resulted in a non-significant trend to lower perinatal mortality: treated 4 of 111 (3.6%); untreated 10 of 118 (8.5%). Those most at risk of perinatal loss were further identified by subgroup analysis as principally women over the age of 25 years and overweight for height. There was a significant reduction in macrosomia (birthweight >4.0 kg) at birth from 13.1% in the untreated group to 4.3% in the insulin-treated group.2
It became practice for treatment with prophylactic insulin to be offered. After this practice was evaluated by such as Gabbe et al,3 it was reported that the recognition and treatment of gestational diabetes was in fact associated with a lower perinatal mortality than that of the hospital population. Further uncontrolled studies of insulin therapy offered prophylactically by Coustan and colleagues4 in the US suggested that birthweight was very sensitive to the regime of once-daily prophylactic insulin, but women managed solely on dietary regimes had an expectation of birthweight similar to women with GDM who were untreated. Unfortunately these studies were observational rather than prospective controlled trials and it is likely that selection bias influenced the reported outcomes.
One group working in Italy, using the US diagnostic criteria, identified an apparent increase in the frequency of neonatal complications, including macrosomia, congenital abnormalities, perinatal mortality, and prematurity, as well as maternal complications such as pre-eclampsia and cesarean section in women who had a normal 100-g OGTT but who had intermediate levels of 2-hour plasma glucose within the “normal range”. This group identified 16% of their population as being at increased risk of typical diabetes-related morbidity rather than the expected 3–4%.5 Further studies suggested that one abnormal value rather than two could be associated with an adverse outcome of pregnancy.6
In the late 1980s and early 1990s, the idea became more widespread that the adoption of these diagnostic criteria of impaired glucose tolerance using the World Health Organization (WHO) criteria or one abnormal value using the National Diabetes Data Group (NDDG) criteria might very well be over-diagnosing non-existent medical problems and involving women in potentially dangerous therapeutic intervention programs, and at the same time labeling them as having a high-risk pregnancy when in fact they did not.7 On the other hand, there was evidence that rates of macrosomia, pre-eclampsia, and cesarean section rose in women with milder degrees of abnormal glucose tolerance in a dose–response manner.8 This was clearly an unsatisfactory situation and in response three large-scale trials were established, the first to identify the range of adverse outcomes which might be associated with untreated mild gestational diabetes (the Hyperglycemia and Adverse Perinatal Outcome [HAPO] trial). Two double blind studies, one performed principally in Australia (Australian Carbohydrate Intolerance Study in Pregnant Women [ACHOIS])9 and one in the US (the Maternal Fetal Medicine Units [MFMU] network randomized controlled trial), were designed to identify any adverse outcomes of untreated mild GDM, but also to evaluate any benefit of treatment if adverse outcomes were present in the untreated groups.
EVIDENCE FOR TREATMENT: INTERVENTION TRIALS
The results of the HAPO trial have been discussed in chapter 6 and suggest that in untreated women with mild gestational diabetes there is an increase in each of the primary study outcomes associated with increasing fasting glucose levels and increasing 1- and/or 2-hour glucose levels. These primary outcomes were cesarean delivery, increased birthweight, neonatal hypoglycemia, and fetal hyperinsulinism, detected by cord blood c-peptide measurements. The HAPO study is an observational study and therefore cannot be used to produce guidance on treatment, but it can certainly relate to appropriate cut-offs for the interpretation of glucose tolerance testing in pregnancy.
ACHOIS trial
The ACHOIS trial is the first reported trial of treatment of gestational diabetes with a double blind design in which half the women who were randomized (n = 510) were not told that they had impaired glucose tolerance/ gestational diabetes, nor were their medical attendants aware of this. The headline findings of this trial were that identification and treatment of gestational diabetes was associated with a relative risk of 0.33 (95% CI 0.14–0.75) of serious perinatal complications taken as a composite measure falling from 23 of 510 (4%) to 7 of 490 (1%). Induction of labor was more common in the intervention group as many units had a policy of elective induction with a diagnosis of gestational diabetes. The proportion of large for gestational age babies was 68 of 490 (13%) in the treated group and 115 of 510 (22%) in the routine care group (RR 0.62, 95% CI 0.47–0.81). Cesarean delivery rates were identical in the two groups.
The study included various measures of psychologic well-being to determine if patients’ knowledge of their diagnosis of gestational diabetes leads to adverse out-comes. In fact, there was a general improvement in mood and well-being in the treated group, with a halving in the incidence of postnatal depression: 23 of 490 (8%) versus 50 of 510 (17%) (RR 0.46, 95% CI 0.29–0.73).
The ACHOIS trial was subjected to a certain amount of criticism, not least because the total incidence of adverse perinatal outcomes was that which was expected when combining the treated and untreated groups. This raised the possibility that the differences seen were a chance finding. It is more likely that this general benefit was seen as a result of a Hawthorne effect.10 The most likely explanation for the low rate of complications overall was that the median 2-hour value in the women randomized in the ACHOIS trial was at the low end of the impaired range at 8.6 mmol/L (155 mg/dL). It was, however, a well-conducted trial with a positive endpoint, suggesting that treatment in mild gestational diabetes is beneficial and should be recommended.
MFMU Network Trial
The recently reported MFMU trial has a similar double-blind methodology to the ACHOIS trial,11 but uses different diagnostic criteria for mild gestational diabetes. These were a fasting glucose level of less than 5.3 mmol/L (<95 mg/dL) and after a 100-g load two or more hourly glucose measurements above the following thresholds: 1 hour 10.0mmol/L(180mg/dL); 2 hours 8.6mmol/L (155mg/dL); and 3 hours 7.8mmol/L (140mg/dL). The primary endpoint was a composite outcome of features associated with maternal hyperglycemia, which included perinatal death, neonatal hypoglycemia, hyperbilirubinemia, hyperinsulinemia, and birth trauma. There was no significant difference in this composite primary outcome (treated group n = 485, 149 of 460 [32.4%]; control group n = 473, 163 of 440 [37.0%]; RR 0.87 [97% CI 0.72–1.07]; p = 0.14). The study cohorts included no perinatal deaths. The study did, however, report significant reductions in a series of important secondary out-comes, mean birthweight was approximately 100 g less in the treatment group and the number of large for gestational age infants was significantly reduced in the treatment group: 34 of 477 (7.1%) compared to 66 of 454 (14.5%) in the control group; RR 0.49 (97% CI 0.32–0.76); p < 0.001. Cesarean delivery was significantly reduced (26.9% in the treatment group compared to 33.8% in the control group) and shoulder dystocia was also significantly reduced (7 [1.5%] versus 18 [4%], respectively; RR 0.37 [97% CI 0.14–0.97]; p 0.02).
In both this study and the ACHOIS trial reported above there was a significant reduction in the frequency of pre-eclampsia.
Taken together these two important studies suggest that treatment of mild GDM is likely to improve the outcome of pregnancy to an extent to make it worthwhile.
TIMING OF INTERVENTION
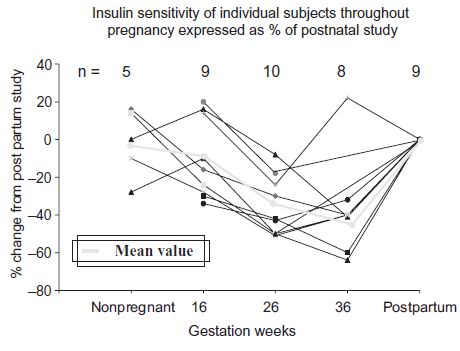
Gestational diabetes is a disorder of the second half of pregnancy in the majority of cases, and probably for most women only the third trimester. The endocrine-induced insulin resistance (the inverse of insulin sensitivity) of pregnancy, which would tip susceptible women over into gestational diabetes, is established by about 26 weeks in most women (Fig. 7.1).
It is the case, however, that insulin sensitivity in pregnancy can be manipulated both up and down by dietary choices, and in those women who are considered susceptible to gestational diabetes, particularly those with preexisting obesity, the adoption of a healthy eating pattern from early pregnancy may be prophylactic against gestational diabetes later in non-diabetic women. We and others have shown that a high carbohydrate, low glycemic index (GI) diet is associated with enhanced insulin sensitivity in later pregnancy.12 Such a dietary approach has no known adverse effects, and when applied by the DIAGEST group of Romon and colleagues (Table 7.1),13 the percentage of large for gestational age babies in women with gestational diabetes managed on diet is inversely proportional to the quintile of carbohydrate intake. A further theoretical advantage of the low GI diet is the abolition of relative ketonemia seen in late pregnancy on a relatively high GI diet (Fig. 7.2). This is important because of the continuing concern that maternal ketonemia in late pregnancy might be capable of inducing neurodevelopmental handicap, including a lowering of IQ.14
In contrast, one non-randomized cohort study reported lower requirements for insulin, and lower rates of cesarean sections and newborn macrosomia with a low carbohydrate regime.15
Fig. 7.1 I nsulin sensitivity of individual subjects and mean throughout pregnancy expressed as a percentage of postnatal study. Negative percentage change represents increasing insulin resistance. (Modified from Stanley et al. with permission from Br J Obstet Gynaecol 1998;105:756–9.)