Fig. 3.1
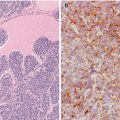
Oncocytic (Hürthle cell, oxyphilic) tumour with clear cell features. Benign (a) and malignant oncocytic follicular tumours have abundant granular eosinophilic cytoplasm due to the accumulation of innumerable abnormal mitochondria (b). Widely invasive biphasic oncocytic clear cell carcinoma (c) in which the lower half of the cytoplasm of tumours cells is oncocytic, whereas the upper half is clear, as a consequence of the swelling of the abundant, mitochondria (d) (Electron micrographs: courtesy of Andrés Beiras Iglesias, Santiago de Compostela, Spain)
Oncocytic (Hürthle Cell, Oxyphilic) Carcinoma Negative for TTF-1 and Thyroglobulin
A few cases of benign and malignant oncocytic tumours negative for TTF-1 and thyroglobulin have been described [5]. The case we selected is from a 76-year-old female with a history of long-standing goitre that has started growing fast causing slight dysphagia and apparently invading the local structures of the neck. Total thyroidectomy and left lymphadenectomy were performed (Courtesy of Ligia Castro, Coimbra, Portugal).
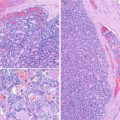
The left lobe was almost totally occupied by a large, poorly circumscribed tumour that invaded the anterior and posterior margins of the specimen (Fig. 3.2). The tumour is composed of cells organized in a solid-patterned fashion. The cells have abundant cytoplasm at variance with the small size of the normal follicular cells; mitoses are scarce. Despite the papillary areas, the nuclei are not PTC type (Fig. 3.3). Angio- and perineural invasion as well as prominent lymphoid infiltration are present. Neither necrotic foci nor node metastases were observed.
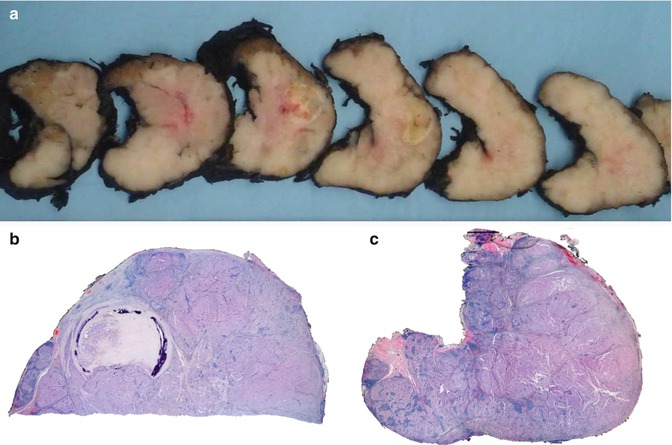
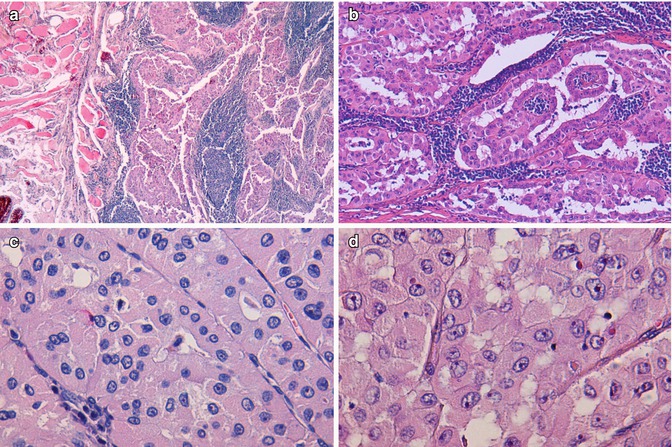

Fig. 3.2
Oncocytic (Hürthle cell, oxyphilic) carcinoma negative for TTF-1 and thyroglobulin. Multinodular lesion with a central fibro-calcified nodule (a, b) and with lymphoid infiltrate (c)

Fig. 3.3
Oncocytic (Hürthle cell, oxyphilic) carcinoma negative for TTF-1 and thyroglobulin. Tumour cells infiltrating skeletal perithyroid muscle (a). Marked lymphocytic infiltration (b) Hürthle cells with abundant finely granular cytoplasm and round to oval nuclei with prominent nucleoli (c, d). Scattered cells with irregular nuclei are not sufficient for the diagnosis of papillary carcinoma (d)
The neoplastic cells are negative for TTF1 and thyroglobulin (Fig. 3.4). Succinate dehydrogenase subunit A protein (SDHA) , a mitochondrial marker, is strong and diffusely positive (Fig. 3.4). The most curious feature of the tumour regards the absence of immunoreactivity for TTF1, thyroglobulin and calcitonin . These unexpected negative findings prompted us to explore the possibility of a metastatic carcinoma that we have ruled out after an exhaustive imagiological search as well as a detailed and thorough immunohistochemical study. The carcinoma was classified as widely invasive because there was invasion of the perithyroid tissues and evidence of a central fibro-calcified nodule probably representing the original tumour. At variance with common FTCs in which it is usually easy to separate encapsulated from widely invasive neoplasms, Hürthle cell carcinomas tend to display a multinodular growth pattern without exhibiting an obvious centrally located tumour.
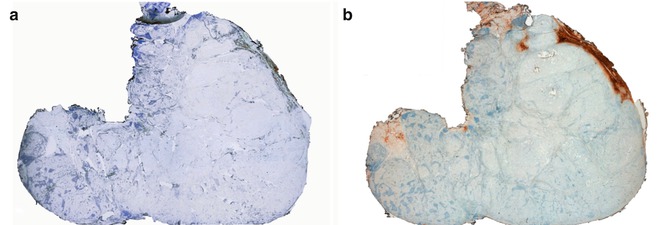
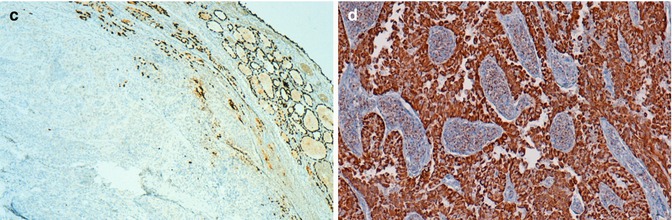


Fig. 3.4
Oncocytic (Hürthle cell, oxyphilic) carcinoma negative for TTF-1 and thyroglobulin. Tumor cells do not show reactivity for TTF-1 (a, c) and thyroglobulin (b) (positive internal control at the periphery). Normal follicles are positive for thyroglobulin and tumor cells disclose a strong positivity for SDHA (mitochondrial marker) (d)
From the clinical standpoint, oncocytic carcinomas negative for thyroglobulin may preclude the use of serum thyroglobulin as a reliable tumour marker during the follow-up of patients harbouring these tumours (see Chap. 2, “Thyroglobulin Negative PTC”) . This case has a number of negative prognostic parameters: incompleteness of surgery, old age of the patient, large size, extrathyroid extension of the tumour and signs of lymphovascular invasion. Molecular data provided no additional information regarding prognosis. The patient was treated with high doses of radioactive iodine and is alive and well 3 years after the diagnosis.
Oncocytic (Hürthle Cell) Tumour with Non-specific Immunoreactivity
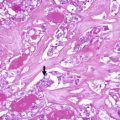
Special care is required in interpreting the results of immunohistochemical analysis of oncocytic cell tumours to avoid false-positive results. Oncocytic cells frequently show non-specific immunoreactivity of the cytoplasm with various antibodies because of high endogenous biotin activity, which may be further enhanced by antigen retrieval procedures [6]. It manifests as coarsely granular staining limited to the cytoplasm that should not be confused with true positivity (Fig. 3.5). This false reactivity may be prevented in many, but not all cases, by endogenous biotin blocking procedures.
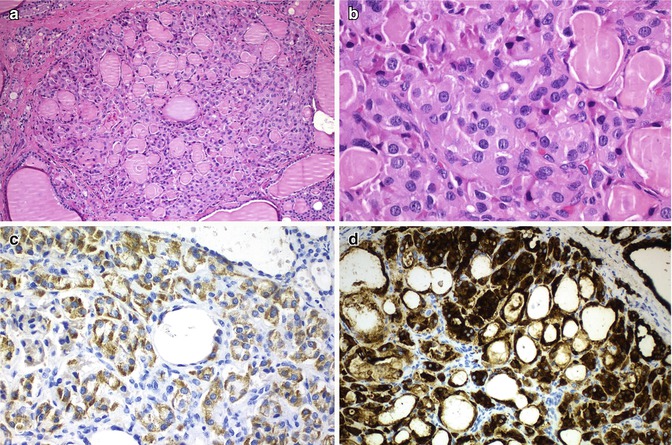

Fig. 3.5
Oncocytic (Hürthle cell) tumour with non-specific immunoreactivity for chromogranin. Oncocytic cells are prone to non-specific staining of the cytoplasm with various antibodies due to their high endogenous activity. It manifests as coarsely granular staining limited to the cytoplasm. In this oncocytic adenomatoid thyroid nodule (a, b) presented in a 46-year-old woman, false cytoplasmic immunostaining for chromogranin was observed (c). Negativity for calcitonin , calcitonin gene-related peptide , carcinoembryonic antigen , synaptophysin and CK20, with positivity for thyroglobulin, thyroperoxidase (d), TTF-1,and CK7 was found in the same tumour cells. mRNA in situ hybridization for thyroglobulin was also positive
Lipid-Rich Follicular Tumours
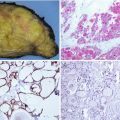
Single cases of benign and malignant follicular neoplasm with intracytoplasmic accumulation of lipid droplets have been documented [7, 8]. In these tumours, the cytoplasm of the vast majority of cells has a characteristic clear, microvesicular, foamy appearance (Fig. 3.6). Focally, a signet ring cell appearance can also be seen. These neoplasms should not be confused with adenolipoma , in which island of mature adipose tissue is mixed with the neoplastic epithelial cells (see Chap. 5, “PTEN Hamartoma Tumour Syndrome ”).
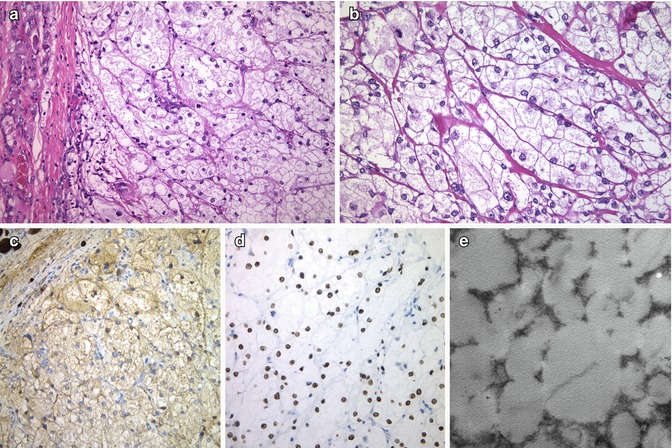

Fig. 3.6
Lipid-rich follicular carcinoma . This well-circumscribed angioinvasive carcinoma shows prominent cytoplasmic clear (foamy) cell features (a, b) and diffuse positivity for thyroglobulin (c) and TTF-1 (d). The ultrastructural study evidenced numerous intracytoplasmic lipid vacuoles (e) (Electron micrograph: courtesy of Andrés Beiras Iglesias, Santiago de Compostela, Spain)
A lipid-rich follicular carcinoma has been described in a 41-year-old woman with McCune-Albright syndrome [8]; the tumour showed 90% of clear cells with capsular and vascular invasion, and immunohistochemistry demonstrated thyroglobulin positivity in the non-clear neoplastic cells, whereas most of the clear cells were negative.
The case illustrated in Fig. 3.6 is an angioinvasive lipid-rich follicular carcinoma displaying a solid pattern of growth, clear cells and diffuse immunoexpression for thyroglobulin, TTF-1 and PAX8, as well as numerous lipid vacuoles at the ultrastructural level.
In addition to lipid accumulation, there are several other mechanisms by which follicular thyroid cells acquire a clear cytoplasmic appearance when examined in haematoxylin and eosin-stained sections. Clear cell follicular tumours (Fig. 3.7) can also be the result of mitochondrial swelling (e.g. oncocytic tumours; see above); dilation of secretory vesicles, the endoplasmic reticulum or Golgi apparatus; and accumulation of glycogen (PAS positive and diastase sensitive), thyroglobulin or mucin [1, 2]. Follicular tumours composed of clear cells should be distinguished from metastatic carcinoma, particularly from the kidney (Fig. 3.7), from clear cell papillary and medullary carcinoma and from normal or tumoural parathyroid tissues (Fig. 3.7). Thyroglobulin and TTF-1 immunoreactivity is helpful for distinguishing clear cell follicular tumours from renal cell carcinoma, medullary thyroid carcinoma and parathyroid tissue. PTC is ruled out by the absence of characteristic nuclear features.
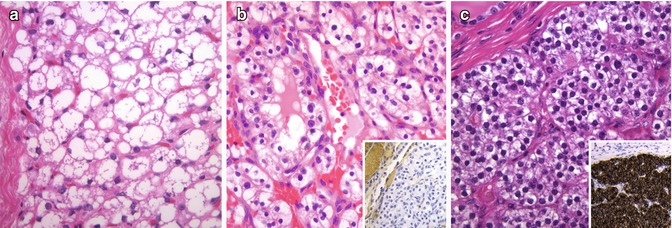

Fig. 3.7
Clear cell thyroid tumours. Benign and malignant follicular tumours with clear cells (a) should be distinguished from metastatic carcinoma, particularly from the kidney (b), and from parathyroid tissue (c). Thyroglobulin and TTF-1 are negative in renal cell carcinoma (b) and parathyroid tissue (c). In this intrathyroidal parathyroid adenoma, strong positivity for PTH can be observed (c, inset)
Follicular Tumours with Signet Ring Cells
In routine sections , this variant of follicular cell tumour is characterized by cells with large intracytoplasmic vacuoles that displace and compress the nucleus to the side [1, 2] (Fig. 3.8). Usually, signet ring cells alternate with others having more conventional features, contain large cytoplasmic vacuoles lined by microvilli and are strongly reactive for thyroglobulin [9] (Fig. 3.8). Less commonly, they may also be positive for mucin stains, a fact that has led to the designations of signet ring cell mucinous adenoma and mucin-producing microfollicular adenoma. In rare cases, large intracytoplasmic lipid vacuoles can also produce a signet ring cell appearance. The presence of signet ring cells is not a feature of malignancy by itself. The diagnosis of follicular carcinoma rests on the identification of capsular and/or vascular invasion. Signet ring carcinoma may be mistaken for metastatic carcinoma from the breast or stomach, which can be excluded by their negativity for thyroglobulin and TTF-1.
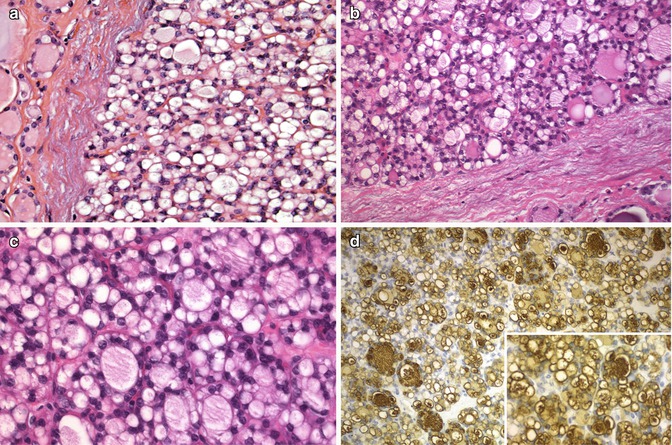

Fig. 3.8
Follicular tumours with signet ring cells. The cells in both adenomas (a, b) reveal large cytoplasmic vacuoles that displace the nuclei laterally, leading to a signet ring configuration. Signet ring cells alternate with follicular structures (c). Cytoplasmic vacuoles, whether small or large, are strongly immunoreactive for thyroglobulin (d)
Atypical Adenoma (Including Spindle Cell Adenoma)
Some pathologists use the wastebasket term of atypical adenoma to designate any follicular adenoma that looks peculiar or worrisome due to the presence of nuclear atypia (Fig. 3.9), high cellularity (cellular or hypercellular adenoma), spindle cells (spindle cell adenoma) (Fig. 3.9), a thick capsule, mitotic activity or necrosis without capsular or vascular invasion [1, 2]. In some adenomas, scattered huge, irregular and hyperchromatic nuclei tend to occur in cluster (adenoma with bizarre nuclei). The bizarre appearance of such nuclei is likely due to cell degeneration, the so-called endocrine atypia, and should not be taken, by itself, as a sign of malignancy. These worrisome features force us to make a meticulous examination of the entire capsule. If there is no invasion, the tumour is clinically benign and the term atypical follicular adenoma is not appropriate.
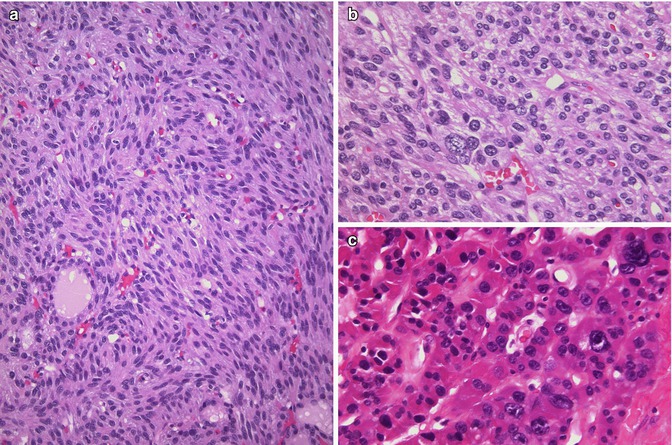

Fig. 3.9




Atypical adenoma (including spindle cell adenoma). Atypical adenoma is a vague and imprecise designation used to refer to any follicular adenoma that looks peculiar or worrisome due to the presence of spindle cells (a), nuclear atypia (b, c), a thick capsule, mitotic activity or necrosis without capsular or vascular invasion. The follicular nature of spindle cell adenoma (a, b) can be confirmed by its immunoreactivity for thyroglobulin and TTF-1. Tumour cells with giant nuclei (bizarre nuclei) (b, c) are not a sign of malignancy, indicating probably a degenerative phenomenon
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree