Chromosomal location
Gene
Thyroid tumour/s
Additional lesions
19p13.11
GRIM-19 (NDUFA13)
Multifocal Hürthle cell (oncocytic) adenoma, PTC and/or FTC
19p13.2
TCO (unknown)
Multifocal Hürthle cell (oncocytic) adenoma, PTC and/or FTC
1q21
PTCPRN (fPTC/PRN1) (unknown)
PTC
Papillary renal neoplasia
9q22.33
FOXE1 (TTF-2)
PTC
9q34.13
TTF-1 (NKX2-1)
MNG, PTC
12q14.2
SRGAP1
PTC
10q25.3
HABP2
PTC
8p23.1-p22
Unknown
MNG, follicular adenoma, PTC
2q21
NMTC1 (unknown)
PTC (classical and follicular variant)
6q22
Unknown
PTC
8q24
Unknown
PTC
Table 5.2
Syndromic familial non-medullary thyroid carcinomas
Syndrome | Gene and location | Thyroid tumour/s | Main additional lesions |
Familial adenomatous polyposis | APC 5q22.2 | Cribriform-morular variant of PTC | Multiple colonic adenomatous polyps, colorectal cancer, desmoid tumours and osteomas (Gardner syndrome), medulloblastoma (Turcot syndrome), hepatoblastoma, congenital hypertrophy of the retinal epithelium |
PTEN hamartoma tumour syndrome (Cowden syndrome and Bannayan-Riley-Ruvalcaba syndrome) | PTEN 10q23.31 | MNG, multiple adenomatous nodules (“microadenomas”), follicular adenoma, lipoadenoma, PTC, FTC, lymphocytic thyroiditis, foci of adipose infiltration, C-cell hyperplasia | Fibrocystic breast disease, breast hamartoma, breast cancer, trichilemmomas, keratosis, oral papillomas, cutaneous papules, pigmentation of penis, endometrial carcinoma, upper and lower gastrointestinal (hyperplastic, adenomatous, hamartomatous, lipomatous, ganglioneuromatous and inflammatory) polyps and cancer, macrocephaly, Lhermitte-Duclos disease, mental retardation, autism, renal carcinoma, glycogenic acanthosis, storiform collagenoma, lipomas |
DICER1 syndrome | DICER1 14q32.13 | MNG, differentiated thyroid carcinoma | Pleuropulmonary blastoma, cystic nephroma, ovarian Sertoli-Leydig cell tumours, gynandroblastoma, juvenile granulosa cell tumours, pituitary blastoma with infant Cushing disease, nasal chondromesenchymal hamartoma, ciliary body medulloepithelioma, embryonal rhabdomyosarcoma of the cervix, anaplastic sarcoma of kidney |
Werner syndrome (adult progeria) | WRN 8p12 | PTC, FTC, undifferentiated (anaplastic) carcinoma | Prematurely aged facies, scleroderma-like skin changes, cataracts, premature arteriosclerosis, type 2 diabetes, osteosarcoma, soft tissue sarcomas, melanoma, meningioma, myeloid disorders |
Carney complex type 1 | PRKAR1A 17q24.2 | Follicular adenoma, PTC | Skin and mucosal pigmentary abnormalities (blue nevi), atrial myxoma, mucocutaneous myxomas, pigmented nodular adrenocortical disease, large-cell calcifying Sertoli cell tumour, psammomatous melanotic schwannomas, pituitary adenomas |
Due to its rarity and importance for the understanding of thyroid carcinogenesis, this chapter focuses on the characteristics of cribriform-morular variant of PTC which is usually associated with FAP but can also be sporadic, as well as on tumours arising in the context of PHTS and DICER1 syndrome . The peculiar morphological and immunohistochemical characteristics of some of such thyroid tumours allow pathologists to play a crucial role in the recognition of the respective hereditary conditions.
Cribriform-Morular Variant of Papillary Thyroid Carcinoma
Cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC) is the peculiar form of thyroid carcinoma associated with familial adenomatous polyposis (FAP) that can also occur as a sporadic tumour [3–5]. The name “cribriform-morular variant” of PTC was coined by our group in 1999 to describe the sporadic morphological counterpart of FAP-associated familial non-medullary thyroid carcinoma [5]. About 60% of the cases are associated with FAP, and in these cases, the diagnosis of thyroid carcinomaprecedes that of FAP in up to 40%. CMV is a tumour with a striking prevalence in women (female-to-male ratio of 61:1) in contrast to a female-to-male ratio of colonic tumours of about 1:1 in FAP. The mean age of primary diagnosis is 26.5 years (median 26, range 8–61 years) with no significant difference between FAP-associated and sporadic CMV of PTC [6].
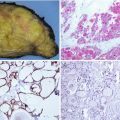
Most tumours are well-circumscribed or encapsulated, white to tan, fleshy and solid, although cystic areas can be seen in some cases. In FAP patients, the tumours are more often multifocal and/or bilateral than in the sporadic cases (63% versus 13%) (Fig. 5.1).
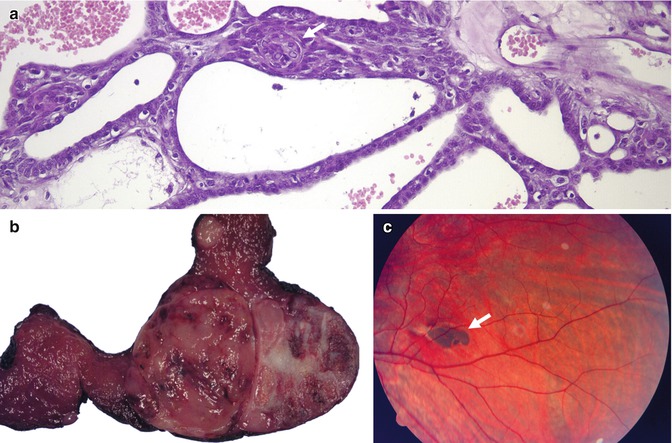

Fig. 5.1
Familial adenomatous polyposis (FAP) -associated cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). CMV is characterized by a combination of cribriform pattern of growth and morular (arrow) structures (a). FAP-associated CMV of PTC is often multiple and bilateral (b). In these patients, the germline mutations usually occur in exon 15 before codon 1220; curiously, the same codons are implicated in the development of thyroid carcinoma and congenital hypertrophy of the retinal pigment epithelium (CHRPE). Fundoscopy showing CHRPE (arrow) is therefore a good way to confirm the hereditary nature of the CMV of PTC before the genetic study (c, courtesy of Rosario Touriño Peralba, Santiago de Compostela, Spain)
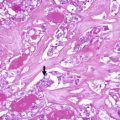
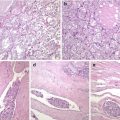
CMV of PTC is usually encapsulated or well circumscribed and partially divided in lobules by sclerotic septa. It is characterized by a blending of cribriform, follicular, papillary, trabecular and solid patterns of growth , with morular (squamoid) structures [4, 5] (Figs. 5.2, 5.3 and 5.4). Cribriform areas are formed by anastomosing bars and arches of cells in the absence of fibrovascular stroma and typically merge with small tubular follicles. Both large follicles (cribriform pattern) and tubular structures are frequently devoid of colloid or may contain foamy and/or multinucleated histiocytes. Nonarbourizing papillary and pseudopapillary elements are usually focal and lined by columnar cells. Areas with trabecular arrangement of plump spindle cells with the nuclei aligned perpendicular to the long axis are reminiscent of the so-called hyalinizing trabecular tumour (Fig. 5.4). The tumour cells are cuboidal or tall, with frequent nuclear pseudostratification and abundant amphophilic to oxyphilic cytoplasm. The nuclei are usually hyperchromatic, variably displaying the nuclear features of classic PTC. In the solid areas, tumour cells become oval to plump and spindly and commonly show nodular whorls resembling squamoid nests (morules). Morules lack keratinization and show scattered or groups of cells with peculiar nuclear clearing, characterized by replacement of the chromatin by a homogeneous, light eosinophilic material throughout the entire nucleus (Fig. 5.5). These morules are also seen interspersed among the other trabecular and cribriform areas, but they can be difficult to find in some cases. Alcian blue (pH 2.5) and Mayer’s mucicarmine stains are negative. Psammoma bodies are rarely found [7]. Foci of necrosis can be seen, and capsular and vascular invasion were reported in about 40% and 30% of cases, respectively [4–10].
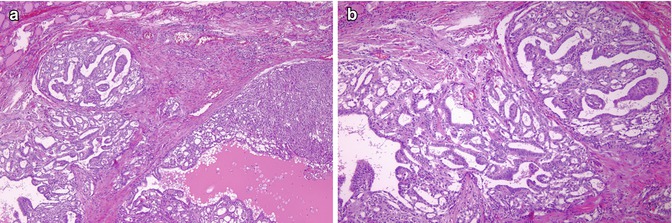
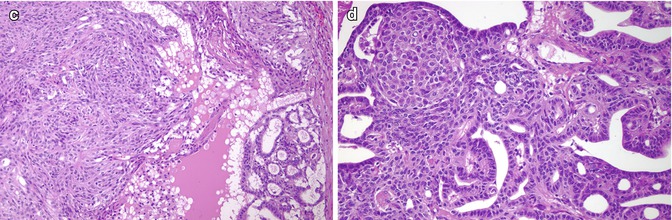
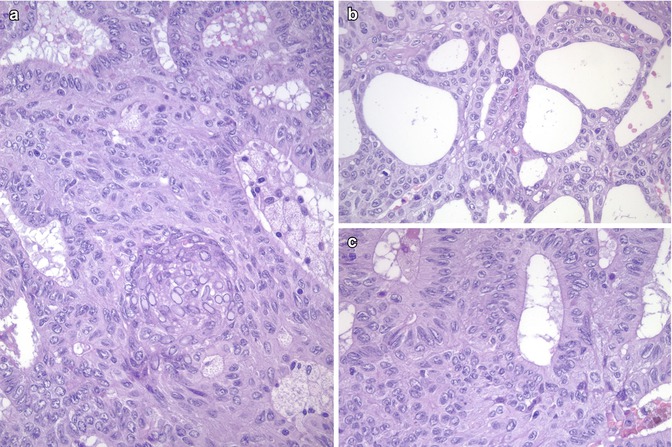
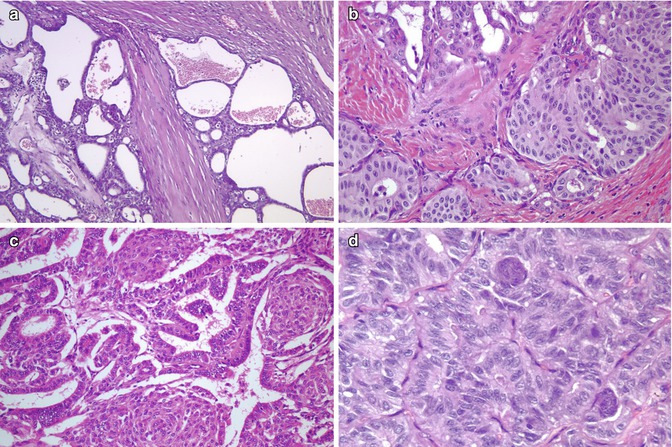
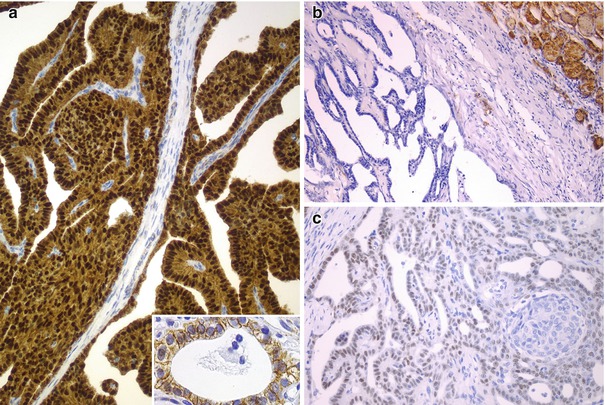


Fig. 5.2
Cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). This sporadic case is from a 31-year-old female. CMV of PTC is usually well circumscribed and partially divided in lobules by sclerotic septa (a). It is microscopically characterized by an intricate blending (a) of cribriform, follicular, papillary (b) and solid (c) patterns of growth, with morular (squamoid) structures (d). Colloid is rarely seen in the follicular lumina

Fig. 5.3
Cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). Familial adenomatous polyposis-associated case of CMV of PTC observed in a 29-year-old female. The tumour showed the typical admixture of solid (a), cribriform (b) and follicular architecture (c), as well as morular structures (a). The nuclei of tumour cells are not particularly clear but show a round to oval shape with prominent grooving (a–c) and pseudoinclusions. In the morular structure shown in (a), numerous biotin-rich, optically clear nuclei can be identified

Fig. 5.4
Cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). CMV of PTC may show a highly variable combination of cribriform, papillary, solid, trabecular and morular growth patterns (a–d). In some cases the cribriform growth pattern may mimic mammary cribriform carcinoma (b). A trabecular pattern reminiscent of hyalinizing trabecular adenoma of the thyroid can also be seen (d)

Fig. 5.5
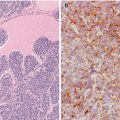
Immunohistochemical features of the cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). Beta-catenin is the hallmark of the CMV of PTC. Immunohistochemistry demonstrates a change in beta-catenin expression from the membranous pattern found in normal follicular cells (inset) to the cytoplasmic and nuclear staining in tumour cells of CMV of PTC (a). Neoplastic cells may be totally negative for thyroglobulin (b), but they are consistently positive for TTF-1 (c)
Additional areas with an adamantinous-like pattern of growth, atypical mitotic figures and hyaline globules (thanatosomes) were detected in one case with neuroendocrine differentiation and aggressive behaviour [6]. Another rare case of CMV of PTC combining an adenoid cystic carcinoma-like growth pattern due to deposition of basement membrane-like material has also been reported [11].
Tumour cells may be focally positive or totally negative for thyroglobulin, but they are consistently positive for thyroid transcription factor-1 (TTF-1) and consistently negative for calcitonin and CK20 and always show strong nuclear and cytoplasmic reactivity for β-catenin [4–21] (Fig. 5.5). A rare case with tumour cells positive for TTF-1 and also positive for chromogranin and synaptophysin in 40% of the tumour mass but negative for thyroglobulin and calcitonin was reported by our group [6]. CMV of PTC is characteristically positive for oestrogen receptors α and β and progesterone receptor and can show focal positivity for androgen receptor; these features seem to be the result of crosstalk between WNT/β-catenin and oestrogen signalling pathways and are probably related to their female predominance (Fig. 5.6). The proliferative (Ki-67) index is generally less than 5% but can reach up to 60% in aggressive (poorly differentiated) tumours [6, 9]. A rare case with positivity for β-human chorionic gonadotropin has been reported [19]. No reactivity was found for carcinoembryonic antigen (CEA) , CA125, epidermal growth factor receptor, Wilms tumour protein (WT1), c-kit (CD117), p63 or calretinin.
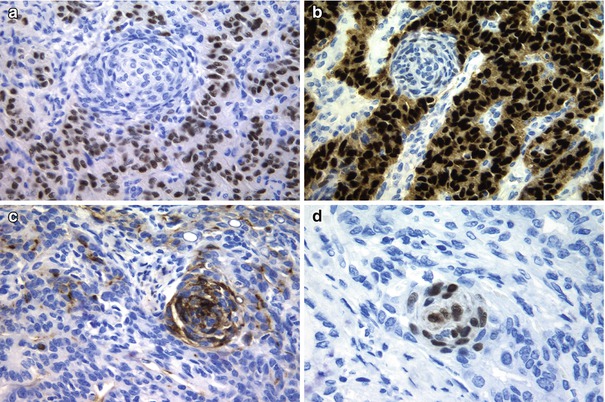

Fig. 5.6
Immunohistochemical features of the cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). Tumour cells in CMV of PTC are positive for oestrogen (a) and progesterone receptors (b), but these immunomarkers are negative in the morular component. The morular structures are selectively stained for CD10 (c) and CDX2 (d)
The morular component of CMV has a distinctive immunohistochemical profile with positivity for β-catenin , CKs AE1/AE3, E-cadherin, bcl-2, cyclin D1 , CA19.9 and galectin-3 , as well as negativity for TTF-1, thyroglobulin, calcitonin , vimentin CEA, CK7, CK20, CK 34βE12 and CA125. The characteristic optically clear nuclei contain biotin; due to their high endogenous biotin content, false-positive reactions in the nuclei of morular cells are highly probable whenever using immunohistochemical systems containing (strep) avidin [5]. CD10 is a useful tool in identifying morules not only in the CMV of PTC but also in all biotin-rich optically clear nuclei family tumours [22], all of them sharing alterations in the WNT/β-catenin signalling pathway (Fig. 5.6). Interestingly, CDX2 , an intestine-specific homeobox gene transcription factor, is also selectively expressed in the CMV morules [23] (Fig. 5.6).
In contrast to morular structures, the true foci of squamous metaplasia lack nuclear staining for β-catenin, are negative for Bcl-2 and show numerous S100 protein-positive dendritic cells, especially at the periphery [14] (Fig. 5.7).
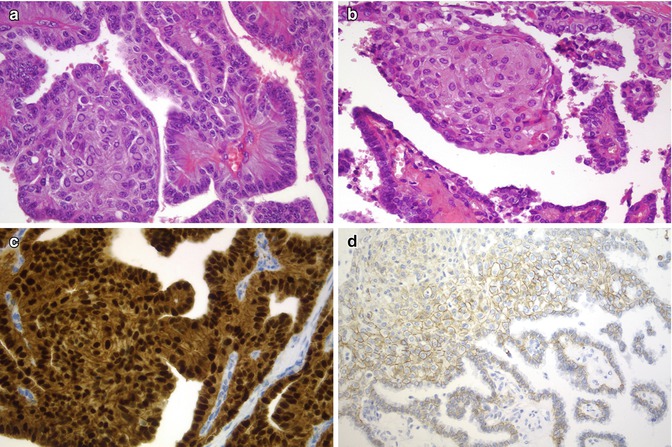

Fig. 5.7
Morules and squamous metaplasia in papillary thyroid carcinoma (PTC). In this figure, a case of CMV of PTC (a) is compared with a classic PTC with squamous metaplasia (b). While in CMV both the nucleus and cytoplasm of all tumour cells are strongly stained for beta-catenin (c), there is only a limited staining, with membrane pattern, in classic PTC cells (d)
The peculiar cribriform pattern of growth along with the positivity for oestrogen and progesterone receptors, as well as the tubulo-glandular pattern of the CMV of PTC, can mimic metastatic carcinoma from the breast and colon , but positivity for TTF-1, which is always positive in CMV of PTC , can help in the differential diagnosis. Columnar cell variant of PTC shows significant morphologic overlap with the CMV of PTC (nuclear pseudostratification, solid areas and elongated, empty follicles resembling tubular glands); however, tumour cells in the columnar cell variant have nuclear hyperchromasia, may contain supranuclear and subnuclear cytoplasmic vacuoles reminiscent of those of early secretory endometrium and are positive for thyroglobulin.
Fine needle aspiration biopsy (FNAB) is an effective method for diagnosing thyroid malignancy and is even able to make a diagnosis of CMV of PTC [8, 10, 12, 17–19, 21, 24–26] (Figs. 5.8 and 5.9). The smears are typically hypercellular, disclosing cohesive papillary fragments lined by epithelial cells with nuclear stratification and nucleomegaly as well as solid flat monolayers. Tumour cells are usually tall and columnar with obscure ground-glass nuclei and abundant spindle cytoplasm with distinct cell borders (Fig. 5.8). Typical nuclear features of classic PTC are commonly seen, but prominent nucleoli are absent (Fig. 5.9). Frequent acinar/cribriform formations devoid of colloid and less common dense cellular (squamoid) morules composed of epithelial cells in a tightly haphazard or whorly arrangement can be seen (Figs. 5.8 and 5.9). The background is generally clean with no multinucleated giant cells, lymphocytes or colloid. Although the detection of cribriform fragments, cellular morules and columnar cells in cytological samples is highly indicative of CMV of PTC, there is much overlap between the architectural and nuclear features of conventional PTC and CMV of PTC. Positive nuclear β-catenin immunostaining plays a definitive role for diagnosing a CMV of PTC [17, 24, 25].
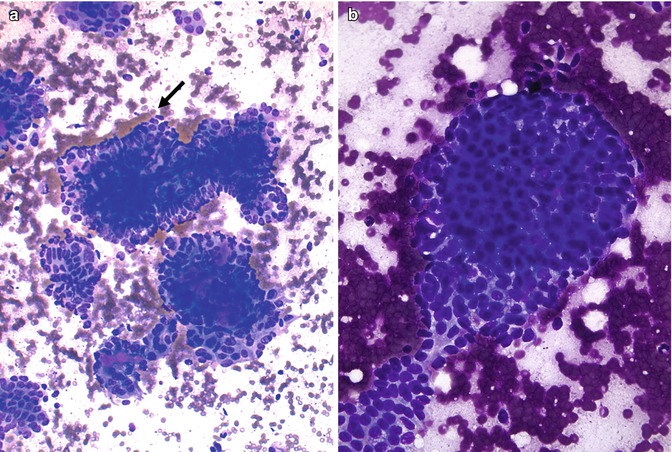
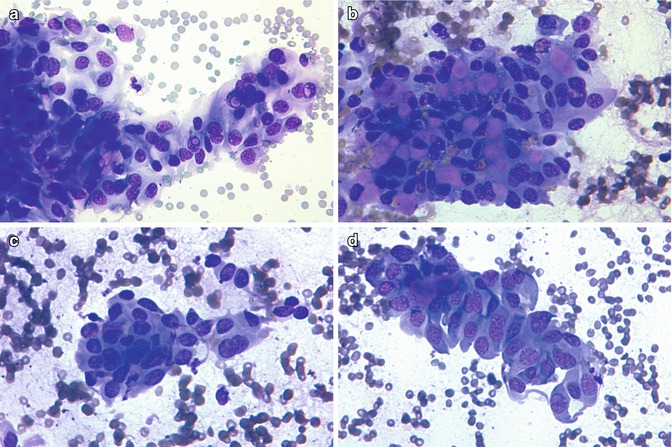

Fig. 5.8
Cytologic features of the cribriform-morular variant (CMV) of papillary thyroid carcinoma (PTC). Papillary arrangement with tall, columnar cells at the periphery (arrow) (a, Diff-Quik stain). Sheet of cells with morular arrangement (b, Diff-Quik stain). This case of CMV of PTC is from a 20-year-old woman with a multicentric, bilateral tumour (T3N0M0) with familial history of adenomatous polyposis. Total thyroidectomy was performed. The patient has no evidence of disease at 84 months of follow-up

Fig. 5.9
Cytologic features of the cribriform-morular variant of papillary thyroid carcinoma. Nuclear groves and pseudoinclusions (a). Cluster with hyaline material (b). Morular structure (c). Tall, columnar cells (d). Same case as in Fig. 5.8 (Diff-Quik stain)
In more than 90% of FAP-associated thyroid carcinomas, the tumours have a typical CMV histology. Therefore, clinicians should be alerted to the possibility of FAP whenever a diagnosis of CMV of PTC is made, namely, when there is not a familiar setting.
CMV of PTC is the typical thyroid tumour occurring in FAP , an autosomal dominant disorder caused by a germline mutation in the adenomatous polyposis coli (APC) gene , which is located on 5q21–22. APC gene encodes the tumour suppressor APC protein that acts as an antagonist of the WNT/β-catenin signalling pathway. Overall, somatic mutation of the APC gene appears in more than 30% of the sporadic CMV of PTC examined. Additional somatic APC mutation have been detected in 50% of the tumours of patients with germinal APC mutation. (“Second hit” of the Knudson’s two hit model) [27] (Table 5.3).
Table 5.3
Main somatic mutations in the cribriform-morular variant of papillary thyroid carcinoma
Reference | F/M | FAP/SPO | APC | CTNNB1 | AXIN1 | BRAF | RET/PTC | PAX8/PPARγ | K/N/HRAS | PIK3CA | TERT |
|---|---|---|---|---|---|---|---|---|---|---|---|
Soravia et al. [7] | 3/0 | 3/0 | 1/3a | ND | ND | ND | 3/3b | ND | ND | ND | ND |
Miyaki et al. [28] | 2/0 | 2/0 | 2/2c | ND | ND | ND | ND | ND | ND | ND | ND |
Cetta et al. [29] | 6/0 | 6/0 | 0/6 | ND | ND | ND | 4/5d | ND | ND | ND | ND |
Xu et al. [13] | 5/0 | 1/4 | 0/5 | 5/5e | ND | ND | ND
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|