Upwards of 40% of patient with colorectal cancer develop peritoneal carcinomatosis (CRCPC). Of the 2500 patients reported in the literature, 1000 underwent cytoreductive surgery (CRS) plus hyperthermic intraperitoneal chemotherapy (HIPEC), resulting in median survival of 22 to 63 months. However, level I data from prospective randomized trials are limited. Further trials are indicated to identify peritoneal carcinomatosis in at-risk patients early in the natural history of the disease and confirm the efficacy of multimodality therapy (CRS/HIPEC/systemic therapy) in those with CRCPC amenable to CRS in the modern era of novel targeted and cytotoxic systemic therapy.
- •
Peritoneal carcinomatosis (PC) from colorectal cancer (CRC) treated with chemotherapy alone results in median survival of 5 to 13 months.
- •
Approximately 55% of high-risk patients (patients presenting with synchronous PC, ovarian metastases, perforated primary CRC, and emergency presentation of CRC with bleeding or obstructing lesions) develop PC.
- •
Early PC is generally undetectable by conventional cross-sectional or functional imaging.
- •
Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for early CRCPC results in median survival of 22 to 63 months and 5-year survival of ∼50%.
- •
Efforts are under way to identify patients at high risk for carcinomatosis or those with early peritoneal disease dissemination and to intervene at a time in the natural history of the disease when treatment-related benefit is highest with multimodality therapy, including CRS/HIPEC/chemotherapy.
Introduction: nature of the problem
Peritoneal carcinomatosis (PC) is a frequently occurring event in the natural history of malignancy of colorectal cancer (CRC) origin (upwards of 40% of all patients with CRC), and is associated with marked deterioration in quality of life (QOL) and limited overall survival (OS). Despite advances in early detection of CRC, peritoneal disease spread continues to be a common mode of disease progression, because 8% of patients with CRC have synchronous peritoneal spread of disease at time of primary resection, and up to 25% of patients with recurrent CRC have disease confined to the peritoneal cavity. In about 30% of patients with CRC, peritoneal spread is the main reason for death.
Clinical presentation and course
PC represents a formidable treatment challenge in oncology. Once considered a variant of systemic spread of disease, CRCPC was treated with palliative systemic chemotherapy alone, with surgery reserved only for palliation of disease-related or treatment-related secondary events such as bowel obstruction and ascites. Systemic multidrug chemotherapy has not altered significantly the natural history of CRCPC, as patients suffer disease progression and functional deterioration. This situation is attributable to visceral obstruction, malignant ascites, and cancer cachexia over a limited median survival of 5 to 9 months.
Novel first-line 5-fluorouracil (5-FU)/leucovorin (LV)-based cytotoxic chemotherapeutic regimens to treat metastatic (liver and lung) CRC, including oxaliplatin (FOLFOX) and irinotecan (IFL, FOLFIRI) with or without targeted antibody therapy using bevacizumab (IFL/bevacizumab) or cetuximab (Erbitux), have increased response rates (25%–55%) and median OS (from 12 to 24 months) significantly greater than what has been the benchmark regimen over the past 4 decades (5-FU or 5-FU/LV). However, long-term survival for patients with systemic disease spread remains poor, and the outcomes for patients with advanced disease confined to peritoneal surfaces treated with these modern agents, indeterminate.
Clinical presentation and course
PC represents a formidable treatment challenge in oncology. Once considered a variant of systemic spread of disease, CRCPC was treated with palliative systemic chemotherapy alone, with surgery reserved only for palliation of disease-related or treatment-related secondary events such as bowel obstruction and ascites. Systemic multidrug chemotherapy has not altered significantly the natural history of CRCPC, as patients suffer disease progression and functional deterioration. This situation is attributable to visceral obstruction, malignant ascites, and cancer cachexia over a limited median survival of 5 to 9 months.
Novel first-line 5-fluorouracil (5-FU)/leucovorin (LV)-based cytotoxic chemotherapeutic regimens to treat metastatic (liver and lung) CRC, including oxaliplatin (FOLFOX) and irinotecan (IFL, FOLFIRI) with or without targeted antibody therapy using bevacizumab (IFL/bevacizumab) or cetuximab (Erbitux), have increased response rates (25%–55%) and median OS (from 12 to 24 months) significantly greater than what has been the benchmark regimen over the past 4 decades (5-FU or 5-FU/LV). However, long-term survival for patients with systemic disease spread remains poor, and the outcomes for patients with advanced disease confined to peritoneal surfaces treated with these modern agents, indeterminate.
Relevant anatomy/pathophysiology
The biology of PC is distinctive, unlike hematogenous metastasis. Insights into the natural history of peritoneal tumor dissemination have engendered novel multimodality treatment approaches to this challenging clinical problem. Tumor dissemination across peritoneal surfaces occurs through established mechanisms of direct tumor extension, transcoelomic tumor cell spread in peritoneal fluid, and malignant peritoneal seeding from surgical manipulation of the tumor; this form of malignant disease dissemination can occur in the absence of regional or distant nodal or systemic metastases. Another possibility is hematogenous spread to the peritoneal surfaces, although evidence for this theoretic possibility does not exist.
Confinement of disease to the parietal peritoneal surface, in the absence of systemic metastasis, has served as the basis for undertaking surgical eradication of disease through aggressive surgical cytoreduction, or cytoreductive surgery (CRS). However, surgery alone has not achieved significant improvement in survival in patients with PC, because microscopic or grossly apparent disease inevitably remains after even aggressive CRS.
Therapeutic options
That viable tumor cells become sequestered in avascular intraperitoneal adhesions explains partly the resistance to and ineffectiveness of systemic therapy alone for PC. The presence of an anatomic barrier, the peritoneal-plasma partition, has enabled administration of high local concentrations of chemotherapy at the peritoneal surface in excess of systemically administered agents when drug delivery is intraperitoneal. For example, high-molecular-weight agents such as mitomycin C (334 Da), and oxaliplatin (397 Da) have favorable pharmacokinetic profiles (area under curve (AUC), peritoneal fluid relative to plasma: mitomycin C, 75:1; oxaliplatin, 25:1) permitting dose-dense intraperitoneal therapy over prolonged periods with rapid tissue concentration (in residual tumor deposits and peritoneum), but limited systemic absorption or toxicity. This unique therapeutic approach addresses the problem of systemic chemotherapy resistance and, with its reduced systemic toxicity, provides distinct pharmacologic advantage over systemic drug delivery.
Intraperitoneal hyperthermia, shown to be technically feasible, was integrated into the treatment paradigm of CRS and intraperitoneal chemotherapy for PC to increase tissue penetration, direct killing of free tumor cells, and augment cytotoxicity of the delivered antineoplastic agent. Another advantage is the homogenous distribution of the intraperitoneal chemotherapy. Hyperthermia itself is cytotoxic to tumor cells based on established mechanisms involving inhibition of nuclear matrix-mediated functions essential to DNA replication, transcription, and repair. However, it is the combined antitumor effect of heat and intraperitoneal chemotherapy that serves as the basis for the currently practiced treatment approach to PC. The abdomen in this concept is seen as a localized compartment, and thus CRCPC without metastatic disease is considered locally advanced disease.
Although hyperthermic intraperitoneal chemotherapy (HIPEC) permits high local drug concentrations to exposed peritoneal surface tumors, 1 important limiting factor is the narrow depth of tissue penetration by the delivered cytostatic agent. Depth of drug peritoneal penetration is limited to 3 mm or less from the parietal peritoneal surface. Hence, the efficacy of HIPEC is inversely proportional to the volume of residual disease; thereby, therapeutic benefit is maximized when all grossly apparent disease is resected (complete cytoreduction, CCR0).
This situation makes aggressive cytoreduction logical and imperative, which is conducted with the intent to eradicate macroscopic deposits of tumor and optimize the efficacy of HIPEC in obliterating minimal residual disease. Optimal therapeutic synergy is achieved when intraperitoneal heated chemotherapy is administered immediately after maximal cytoreduction, thereby minimizing trapping of viable peritoneal tumor cells in fibrin and postoperative adhesions, and maximizing kill of tumor cells shed during resection. Adhesions have to be divided during CRS to facilitate uniform distribution of perfusate, maximize direct contact of drug with residual peritoneal tumor cells, and harness the advantage of thermochemotherapeutic antitumor synergism.
PC of CRC origin has long been considered a preterminal condition. A multicenter prospective study determined median survival in this group of patients to be 5 months. A retrospective analysis of more than 3000 patients with CRC reported median OS of 7 months in patients with CRCPC. The trimodality therapeutic paradigm to PC consisting of CRS plus HIPEC followed by systemic chemotherapy has shown promising oncological outcomes.
Clinical outcomes
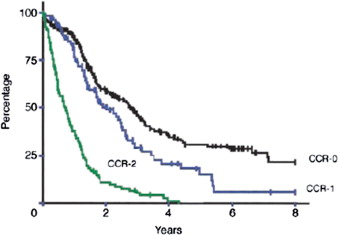
A multicenter registry study of more than 500 patients with CRCPC treated with CRS plus HIPEC reported median OS of 19.2 months, and 3-year and 5-year OS rates of 39% and 19%, respectively. For patients with no macroscopic residual disease after cytoreduction (CCR0), 3-year and 5-year OS was 47% and 31%, with median survival of 32.4 months ( Fig. 1 ), similar to outcomes after complete resection of CRC liver metastases. Treatment with adjuvant systemic chemotherapy after cytoreduction and perioperative thermochemotherapy was an independent predictor of improved survival on multivariate analysis. This study, although retrospective in nature, suggested that improved outcomes are indeed possible with a combined modality treatment approach incorporating CRS, regional/compartmental intraperitoneal chemotherapy with or without adjuvant systemic therapy in patients who could otherwise expect limited survival ranging from 5 to 8 months. OS in another large international registry study was consistent with that reported in previous smaller phase II studies of combined CRS and perioperative HIPEC for CRCPC.
Thus far there is only 1 prospective randomized trial providing level I data, and supporting CRS plus HIPEC for patients with CRCPC. It was a single-institution, randomized controlled trial (RCT) (phase III) trial that showed the superiority of this combined modality approach for patients with colorectal PC over adjuvant systemic therapy, with or without surgical palliation. One hundred and five patients with CRCPC were randomly assigned to receive standard, 5-FU/LV, systemic chemotherapy (standard of care) or CRS plus HIPEC with mitomycin C (35 mg/m 2 at 41° C for 90 minutes). After a median follow-up time of 22 months, median survival was increased significantly in the CRS/HIPEC arm of the study (22.4 vs 12.9 months; hazard ratio = 0.55: 95% confidence interval [CI], 0.32–0.95; P = .032). The analysis was conducted on an intent-to-treat basis and study design required randomization before operation such that only 37% underwent CCR0, which is an imperative if benefit is to be achieved with HIPEC. Another point of contention is the mixed population of high-grade and low-grade CRCs. Subsequently, Verwaal and colleagues reported the long-term outcomes of this trial. At a median follow-up of 8 years (range: 72–115 months), 4 of 51 patients were still alive in the standard therapy arm (2 with and 2 without disease); in the CRS plus HIPEC arm, 5 patients remained alive (2 with and 3 without disease). The median progression-free survival (PFS) was 7.7 months and 12.6 months in the standard therapy and CRS plus HIPEC arms, respectively ( P = .02). The median disease-specific survival was 12.6 and 22.2 months in the standard and CRS plus HIPEC arms, respectively ( P = .028). Based on these data, approximately 5 patients need to undergo CRS plus HIPEC for 1 patient to experience survival advantage at 3 years.
Study subjects in Verwaal and colleagues RCT with extensive carcinomatosis having incomplete CRS also underwent HIPEC. This was the first RCT to report OS benefit in patients with CRCPC treated with CRS and HIPEC when compared with palliative chemotherapy. However, the study used a dated systemic therapy regimen in the form of 5-FU (400 mg/m 2 intravenous [IV] bolus) and LV (80 mg/m 2 IV) administered weekly for 26 weeks or until progression, intolerable toxicity or death, with or without palliative surgery.
Complications and concerns
The absolute OS benefit of ∼10 months in the Dutch RCT conducted by Verwaal and colleagues was offset by considerable treatment-related morbidity (grade 4 morbidity = 45%) and mortality (8%) in the CRS/HIPEC arm. A significant proportion of treatment-associated complications (median operative blood loss 4000 mL; small bowel fistula, 15%; operative site infection, 6%; renal failure, 6%; pancreatitis, 2%) have been hypothesized to be caused by the high dose of intraperitoneal hyperthermic mitomycin C, which was administered in the context of this RCT. Reductions in intraperitoneal mitomycin C doses have been recommended on that basis.
Others have reported significantly lesser treatment-related morbidity (23%–35%) and mortality (0%–4%) with HIPEC using reduced mitomycin C doses. The Dutch RCT reported benefit of CRT with HIPEC for patients with CRCPC, and it challenged the predominant therapeutic nihilism that has been the accepted norm for patients with this disease. The actual contribution of the HIPEC to the observed survival benefit, despite the considerable cost in terms of treatment-related morbidity evident in that trial, remains in question; however, acceptable therapeutic toxicity has been reported in other studies with lower doses of intraperitoneal mitomycin C without apparent compromise in treatment efficacy.
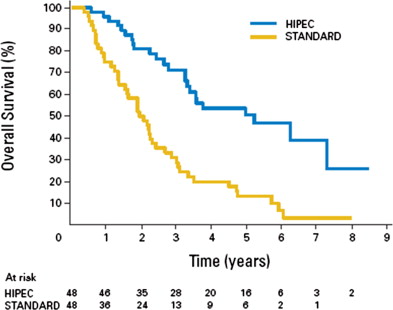
Modern systemic therapy with combination cytotoxic and biologic agents has resulted in unprecedented median OS exceeding 20 months for stage IV CRC. However, the most common mode of distant disease spread in these studies has been hematogenous dissemination. Patients with PC with metastatic disease confined to the peritoneal surface treated with complete (CCR0) cytoreduction and HIPEC have showed median survival exceeding 40 months (range 28–60 months). Based on these data, Elias and colleagues reported on a series that follows patients with CRCPC without systemic dissemination treated with current standard of care systemic chemotherapy regimens of FOLFOX (5-FU, LV, and oxaliplatin) and FOLFIRI (5-FU, LV, and irinotecan). Based on the inability to perform a randomized trial, Elias and colleagues performed a comparative study that case-matched controls to CRS and HIPEC to answer 2 questions. First, what is the natural history of patients with PC treated with modern regimens? Second, do patients treated with CRS and HIPEC benefit in terms of survival? The investigators reported a median survival for the standard group and the CRS/HIPEC group of 23.9 months and 62.7 months, respectively ( P <.05). The 2-year survival was 65% and 81%; 5-year survival was 13% and 51%, respectively ( Fig. 2 ). With the caveat that this was not a prospective randomized trial, patients with CRCPC have benefited from improved multiagent systemic regimens. However, the benefit of modern systemic chemotherapy incorporating combinations of 5-FU, LV, oxaliplatin, irinotecan, capecitabine, bevacizumab and cetuximab for patients with CRCPC without distant metastases is not fully understood. Oxaliplatin-based and irinotecan-based regimens are not without complications. For a full discussion, please see the section on HIPEC with oxaliplatin.
Current standard treatment approach for advanced CRC
Approximately 40% of patients present initially with metastatic CRC, and the majority are treated with palliative multiagent systemic therapy, because the extent of disease precludes complete surgical resection in most of these patients. For more than 40 years a thymidylate synthase-inhibiting fluoropyrimidine analogue, 5-FU, has been the prevailing active cytotoxic chemotherapeutic agent used in combination with a biomodulating agent, LV. However, the clinical benefit with the standard first-line regimen of 5-FU/LV for advanced CRC has been a modest response rate of ∼20%, and median OS ∼12 months.
New agents emerged, which advanced significantly the treatment of metastatic CRC. Various combinations of 5-FU/LV and the topoisomerase I inhibitor, irinotecan, the third-generation platinum analogue, oxaliplatin, and the oral fluoropyrimidine, capecitabine, have nearly doubled the median OS (20–22 months) for patients with advanced disease. Novel targeted agents such as the recombinant humanized monoclonal antibody to vascular endothelial growth factor (VEGF), bevacizumab, and the antibody targeting epidermal growth factor receptor, cetuximab, have expanded the therapeutic options and improved oncological outcomes even further, with median OS exceeding 2 years when all active agents are administered over the course of the patient’s disease.
Three pivotal RCTs showed the superiority of irinotecan (CPT-11) in combination with 5-FU/LV over 5-FU/LV alone (either bolus [Mayo regimen] or infusional 5-FU/LV) in advanced CRC in terms of treatment response rate (RR: 39%–62% vs 21%–34%) and PFS (6.7–8.5 vs 4.3–6.4 months), and in 2 of these studies, OS (14.8–20.1 vs 12.6–16.9 months). These trials established combination weekly infusional 5-FU/LV + irinotecan, 5-fluorouracil and leucovorin (IFL) as a new standard first-line therapy for metastatic CRC in the year 2000.
Three RCTs that compared oxaliplatin with an infusional 5-FU/LV backbone with infusional 5-FU/LV alone as first-line therapy for metastatic colorectal adenocarcinoma reported significant improvement in RR (49%–53% vs 16%–22%) and PFS (7.8–9.0 vs 5.3–6.2 months). The availability of effective second-line (salvage) systemic chemotherapy and modest study group sample size may have limited showing a statistically significant OS benefit with first-line 5-FU/LV + oxaliplatin (OS: 16.2–19.9 vs 14.7–19.4 months).
The North Central Cancer Treatment Group (NCCTG N9741) trial, which compared IFL (control group), irinotecan (125 mg/m 2 ) and bolus FU (500 mg/m 2 ) plus LV (20 mg/m 2 ) on days 1, 8, 15, and 22 every 6 weeks with FOLFOX4, oxaliplatin (85 mg/m 2 on day 1) + infusional FU (400 mg/m 2 IV bolus followed by 600 mg/m 2 continuous 22-hour infusions on days 1 and 2 every 2 weeks) + LV (200 mg/m 2 ), with IROX, irinotecan (200 mg/m 2 ) and oxaliplatin (85 mg/m 2 ) every 3 weeks, established FOLFOX4 as front-line therapy for metastatic CRC. Significant improvement in treatment response, PFS, and OS was reported with FOLFOX4 (RR 45%, PFS 8.7 months, OS 19.5 months) compared with IFL (RR 31%, PFS 6.9 months, OS 15.0 months) and IROX (RR 35%, PFS 6.5 months, OS 17.4 months) in that study.
The FOLOX4 regimen had significantly lower grade 3+ toxicity (including nausea, vomiting, diarrhea, dehydration, and febrile neutropenia) than IFL, but was more commonly associated with neuropathy and neutropenia. This NCCTG trial emphasized the importance of infusional rather than bolus 5-FU administration in combination with irinotecan and oxaliplatin to optimize the tolerability of triple combination therapy. The superior treatment efficacy and safety profile seen with FOLFOX4 in the NCCTG N9741 trial made it the new first-line standard of care for advanced colorectal carcinoma.
Two phase III trials have shown comparable efficacy of FOLFOX and infusional/bolus 5-FU/LV + irinotecan (FOLFIRI) for first-line treatment of stage IV CRC, pointing out that treatment selection largely depends on anticipated adverse event profile (gastrointestinal [GI] toxicity with FOLFIRI vs sensory neuropathy with FOLFOX). FOLFIRI, which incorporates infusional 5-FU, has superseded IFL, which uses bolus 5-FU, because IFL has significant dose-limiting toxicity (diarrhea, dehydration, and myelosuppression) and inferior clinical outcomes. One trial has emphasized the findings of a preceding meta-analysis of 7 randomized phase III trials in advanced CRC, specifically, multidrug triple combination (FOLFOX, FOLFIRI) first-line chemotherapy and administration of all 3 active cytotoxic agents (5-FU, irinotecan and oxaliplatin) over the course of the disease represents the optimal treatment strategy, because it translates into significant OS benefit.
Bevacizumab is a recombinant humanized monoclonal antibody targeting the VEGF-A receptor, which alone has not shown activity against CRC; however, when combined with present-day cytotoxic agents, it has proved highly efficacious. The randomized TREE1 and TREE2 trials compared mixed FOLFOX, bolus 5-FU/LV/oxaliplatin and capecitabine/oxaliplatin (CAPOX) with or without bevacizumab. The addition of bevacizumab enhanced the efficacy of each individual combination by increasing treatment response, PFS, and OS ( Table 1 ).
| Outcome | mFOLFOX | bFOL | CAPOX | |||
|---|---|---|---|---|---|---|
| No Bevacizumab | Plus Bevacizumab | No Bevacizumab | Plus Bevacizumab | No Bevacizumab | Plus Bevacizumab | |
| RR (%) | 41 | 52 | 20 | 39 | 27 | 46 |
| PFS (mo) (95% CI) | 8.7 (6.5–9.8) | 9.9 (7.9–11.7) | 6.9 (4.2–8.0) | 8.3 (6.6–9.9) | 5.9 (5.1–7.4) | 10.3 (8.6–12.5) |
| OS (mo) | 19.2 | 26.0 | 17.9 | 20.7 | 17.2 | 27.0 |
The TREE1/TREE2 trials showed the significant added benefit of bevacizumab (5 mg/kg) when combined with standard first-line FOLFOX for advanced CRC, not only in terms of increased treatment response (52% vs 41%) and PFS (9.9 vs 8.7 months) but also OS (26.0 vs 19.2 months).
The Eastern Cooperative Oncology Group (ECOG) 3200 trial substantiated the added benefit of FOLFOX4 + bevacizumab. Bevacizumab-naive and oxaliplatin-naive patients with advanced CRC previously treated with 5-FU+irinotecan were randomized to receive FOLFOX4, high-dose bevacizumab (10 mg/kg) alone, or FOLFOX4 + bevacizumab. The addition of bevacizumab to second-line FOLFOX significantly increased treatment RR, PFS, and OS ( Table 2 ). Phase III trial data strongly support bevacizumab as an integral component of first-line triple combination therapy (FOLFOX or FOLFIRI) for metastatic CRC. FOLFOX + bevacizumab are now recommended as standard first-line therapy for advanced unresectable CRC by the National Comprehensive Cancer Network. In addition, CAPOX has been added as a potential regimen in this trial, because it is one of the acceptable standard first-line chemotherapy regimens for metastatic disease according to the latest NCCN guidelines.
| Bevacizumab (n = 243) | FOLFOX4 (n = 290) | FOLFOX4 + Bevacizumab (n = 289) | FOLFOX4 + Bevacizumab vs FOLFOX4 P = | |
|---|---|---|---|---|
| RR (%) | 3.0 | 9.2 | 21.8 | <0.001 |
| PFS (mo) | 3.5 | 5.5 | 7.4 | <0.001 |
| OS (mo) | 10.2 | 10.7 | 12.5 | 0.002 |
The need for evidence-based approaches to therapy for combined modality therapy (CRS + HIPEC + systemic therapy)
A standardized, evidence-based approach is lacking for patients with peritoneal surface malignancy from CRC origin. A collaborative trial with surgical quality assurance and modern multidrug systemic therapy incorporating critical assessment of disease burden, determinants of CCR, treatment-related toxicity, QOL, and survival, although imperative, is unlikely to be achieved. The Dutch RCT, although not perfect, has provided the basis for further study, which should use a new reference study arm. If future randomized trials are undertaken for CRCPC origin, then appropriate study selection will be key, which should be limited to selected patients having potentially curable regionally advanced CRC confined to the parietal peritoneal surface.
As systemic therapy for CRC continues to improve, an increasing number of patients fail in the peritoneum despite adequate control of lymphatic and hematogenous dissemination of disease. Peritoneal surface disease is difficult to detect with cross-sectional imaging modalities routinely implemented for this patient population. Patients with peritoneal surface malignancy from GI cancers almost uniformly succumb to advanced locoregional disease in the form of intractable ascites, malignant visceral obstruction, and cancer cachexia. The natural history of peritoneal carcinomatosis from GI malignancies is inexorably lethal, with median OS of approximately 5 months, because patients with disease confined to the peritoneum remain at increased risk of synchronous occult hematogenous metastases. Hence, multiagent systemic therapy is recommended under these circumstances.
Although systemic therapy improves outcome in patients with hematogenous disease spread, improvements are needed to control peritoneal surface malignancy, which is known to be relatively resistant to systemic agents, principally because of the presence of a peritoneal-plasma partition. Moreover, the results of surgical resection alone for peritoneal dissemination of colon cancer have been disappointing given the difficulty in clearing surgically all microscopic disease foci. The infusion of chemotherapy into the peritoneal cavity provides distinct pharmacokinetic advantages. The addition of hyperthermia potentiates the effect of intraperitoneal chemotherapy through antitumor synergism, without systemic drug absorption.
Mitomycin C has been studied most extensively for HIPEC in patients with PC of GI origin. Mitomycin C has also shown consistent pharmacokinetics, favorable toxicity profile, and invariable hyperthermia-facilitated tumor cytotoxicity, which is enhanced under conditions of tumor hypoxia; furthermore, mitomycin C contributes to improved outcomes after optimal cytoreduction. Hence, the delivery of intraperitoneal heated chemotherapy has the advantage of dose-dense regional delivery of cytotoxic agents, with little systemic toxicity.
We have witnessed a milestone development in the treatment of metastatic CRC. Median OS has been doubled through the use of all active agents over the patient’s disease course, from 12 months with 5-FU to nearly 24 months with infusional 5-FU-LV in combination with oxaliplatin or irinotecan, and biologic agents such as bevacizumab and cetuximab. These modern-day multidrug systemic regimens have proven benefit with hematogenous disease spread; however, the role of modern systemic therapy in patients with colorectal carcinoma confined to the peritoneal cavity remains undefined. It is anticipated that these newer agents will prove effective in clearing clinically unapparent systemic circulating tumor cells that place the patient at high risk of distant dissemination of disease after cytoreduction and HIPEC. However, the most common site of metastatic disease in the studies of modern multiagent systemic regimens was hematogenous dissemination. The benefit of modern systemic chemotherapy incorporating 5-FU, LV, oxaliplatin (FOLFOX), bevacizumab, and cetuximab for patients with advanced CRC confined to the peritoneal surface is unknown. Level I evidence strongly supports adding the biologic agent, bevacizumab, to standard first-line triple combination chemotherapy (FOLFOX or FOLFIRI) for advanced CRC (see Table 2 ).
CRS, HIPEC, and systemic chemotherapy are not competitive therapies, and current practice patterns in selected specialty centers reflect the acceptance in some parts of the oncological community that all 3 modalities have a role in the multidisciplinary approach to appropriately selected patients with CRCPC origin. Given that the benefit of current systemic therapy regimens for limited peritoneal disease of CRC origin remains to be determined, an RCT was proposed to compare standard multiagent systemic therapy with combined modality therapy (CRS + HIPEC + systemic therapy; US Military Cancer Institute (USMCI) and American College of Surgeons Oncology Group (ACOSOG)-National Cancer Institute (NCI)/Cancer Therapy Evaluation Program (CTEP) protocol, USMCI 8214/ACOSOG Z6091). The control arm of the RCT was intended to assess response to modern-day systemic agents for documented disease confined to the peritoneal surface. The trial emphasized the importance of exposing patients with metastatic colon cancer to all active agents over the course of disease irrespective of sequence of drug administration. Because current clinical experience suggests contemporary systemic chemotherapy is associated with prolonged survival among patients with CRCPC compared with historical controls, it was postulated that adding CRS to modern systemic chemotherapy regimens could significantly improve oncological outcomes.
The NCI/CTEP protocol USMCI 8214/ACOSOG Z6091 comparing standard systemic therapy with CRS + HIPEC + standard systemic therapy in patients with limited peritoneal dissemination of colon adenocarcinoma recognized the importance of: (1) surgical standardization and quality control; (2) multimodality treatment of patients with CRC having resectable dissemination of peritoneal disease, absent apparent hematogenous or distant nodal disease spread, who are considered suitable candidates for aggressive local-regional therapy: CRS with HIPEC; and (3) the potential challenges of a systemic-chemotherapy-only treatment arm apparent in previous unsuccessful randomized designs. The potential favorable synergy of modern systemic and regional therapies, along with the preference of the larger oncological community to treat advanced CRC with or without CRCPC with upfront systemic therapy, as well as the expectations of patients with CRCPC origin, made it necessary to: (1) include patients who received previous first-line systemic therapy for metastatic CRC; and (2) to allow crossover from the systemic therapy control arm to the multimodality treatment arm at time of disease progression ( Fig. 3 ). Despite this situation, the trial failed to meet accrual goals and closed, calling into question the feasibility of conducting such an RCT to assess the efficacy of combined modality therapy in patients with limited PC of CRC. if definitive level I evidence is absent, some experts in CRS regard combined modality therapy for limited CRCPC as the current standard of care. For example, in France, this therapeutic paradigm has already been incorporated into the French guidelines. In Germany, it will be integrated in to the treatment guidelines as a therapeutic option in 2012.
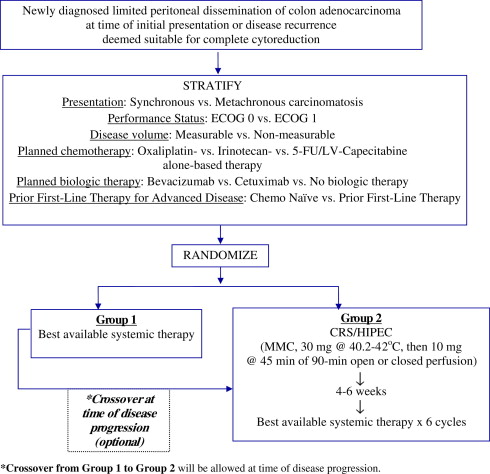
Efforts are under way to identify patients at high risk for carcinomatosis or those with early peritoneal disease dissemination and to intervene at a time in the natural history of the disease when treatment-related benefit is highest with multimodality therapy, including CRS/HIPEC/chemotherapy. Recognizing that ∼55% of high-risk patients (patients presenting with synchronous PC, ovarian metastases, perforated primary CRC, and emergency presentation of CRC with bleeding or obstructing lesions) develop CRCPC, and that early PC is undetectable by conventional cross-sectional or functional imaging, others have taken a different approach to establish level I evidence for CRS/HIPEC in limited PC of CRC origin.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree





