Trial [ref.]
Survival*
Local recurrence
Mastectomy
BCT
Mastectomy
BCT
Median f/u
Milan I [1]
58.8 %
58.3 %
2.3 %
8.8 %
20 year
NSABP BO6 [2]
47 %
46 %
10.1 %
14.3 %
20.8/20.7 year, resp.
Danish [3]
79 %
82 %
3.7 %
2.6 %a
40 month
EORTCb [4]
60.0 %
54.9 %
12.2 %
19.7 %a
13.4 year
Gustave-Roussy [5]
49 %
60 %
9.9 %
15.9 %a
22.7/22.1 year, resp.
NCI/NIH [6]
58 %
53 %
0.0 %
27 %a
18.4 year
Retrospective analyses have demonstrated that the vast majority of local recurrences after BCT are near the site of the original tumor (Table 18.2) [8–11]. Such recurrences are commonly known as “true recurrences” or “marginal misses.” True recurrences are distinguished from recurrences elsewhere in the breast which are referred to as “new primaries” or “elsewhere failures” [12]. True recurrences are much more common than elsewhere failures. Studies have demonstrated that delivery of WB-XRT markedly reduces the risk of true recurrences, but does not appear to impact the low rate of elsewhere failures (Table 18.3) [8–11]. This observation has raised questions regarding the merits of targeting the entire breast with radiation and has suggested a possible role for more limited radiation targeting the breast tissue immediately around the surgical resection cavity.
Table 18.2
Incidence of true recurrences after breast conservation surgery (BCS) in prospective studies (from Dirbas et al., Cancer Biotherapy 2004)
Trial [ref.] | n | BCS | ||
|---|---|---|---|---|
True recurrences | % | Median f/u (months) | ||
Ontario [8] | 421 | 93/108 | 86.1 | 43 |
Swedish (Uppsala-Orebro) [9] | 194 | 8/11 | 72.7 | 27.5 |
Milan III [10] | 273 | 48/56 | 85.7 | 109 |
NSABP BO6 [11] (first events only) | 636 | 40/57 | 70.2 | 125 |
Table 18.3
Incidence of elsewhere failures after breast conservation surgery (BCS) in prospective studies (from Dirbas et al., Cancer Biotherapy 2004)
Trial [ref.] | BCS | BCT | |||
|---|---|---|---|---|---|
n | % | n | % | Median f/u (months) | |
NSABP-B06 [11] | 17/636 | 2.7 | 24/629 | 3.8 | 125 |
Milan [10] | 8/273 | 2.9 | 2/294 | 0.7 | 109 |
Swedish (Uppsala-Orebro) [9] | 3/194 | 1.5 | 1/187 | 0.5 | 31 |
Ontario [8] | 15/421 | 3.6 | 4/416 | 1 | 43 |
This more focused approach to post-surgical breast irradiation has become referred to as accelerated, partial breast irradiation (APBI). With APBI, radiation is targeted to the margin of breast tissue surrounding the lumpectomy cavity rather than the whole breast (hence “partial”). With a smaller target volume, the radiation dose can be escalated and delivered over a shorter time period (hence “accelerated”).
There are currently two general approaches to APBI. The first approach is a BID treatment over 5 days accomplished with either interstitial brachytherapy or intracavitary brachytherapy or limited-field external beam radiotherapy (typically 3 dimensional conformal radiotherapy, or 3D-CRT). The second approach involves a single, very large radiation dose delivered intraoperatively to the lumpectomy cavity margins while the patient is asleep in the operating room (intraoperative radiotherapy, or IORT).
If successful, APBI would be more convenient to patients, would provide less radiation exposure to normal tissues, and could potentially lower treatment costs. General principles of APBI, as well as recent trends in patient selection, surgical technique, and radiation delivery are the focus of this chapter.
Rationale for APBI
Initial randomized studies comparing BCT to mastectomy were designed to compare rates of local recurrence and survival [13, 14]. Retrospective evaluation of these trials demonstrated that approximately 70–90 % of in breast recurrences take place near the original tumor site. Such “true recurrences” usually manifest within 5–7 years of the original resection [15]. WB-XRT markedly reduces true recurrences. New primaries in the breast, or “elsewhere failures,” are less common and do not appear markedly altered by WB-XRT. These elsewhere failures occur at a slow, fairly consistent rate over the patient’s lifetime: they extended beyond the typical 5 to 7-year time frame within which most true recurrences take place. In fact, elsewhere failures appear to be distinct events that temporally mirror the development of new primaries in the contralateral breast. It is important to note that there is some ambiguity in defining a true recurrence vs. an elsewhere failure as there are no strict physical, imaging, or molecular markers that can absolutely distinguish the two. Nonetheless, these general observations regarding true recurrences and elsewhere failures are widely accepted.
The timing, location, probability, and effective reduction with led investigators to initiate trials that directed post-surgical radiation to the breast tissue surrounding the surgical cavity. Due to the smaller target, radiation doses could be escalated. The mathematical tool that enables radiation physicists to adjust dose and schedule this way while achieving the same radiobiologic effect on potential residual cancer cells and normal tissue is the linear quadratic equation [16]. This formula includes tissue-specific variables, the α/β ratio, which represents tumor sensitivity to radiation and normal tissue tolerance by the target organ, such as the breast. In this way, radiation physicists have been able to develop treatment plans that reduced the whole breast radiation treatment time from 6 weeks to a partial breast approach given over 1–5 days with the same anticipated effects on residual microscopic tumor and normal breast tissue.
Early Efforts with APBI
Early attempts at partial breast irradiation were attempted in four trials that used multiple approaches: interstitial brachytherapy catheters; “limited field” external beam radiotherapy; and single fraction IORT. Local recurrence rates in these studies were unacceptably high: two of the studies demonstrated ipsilateral breast tumor recurrence (IBTR) rates of 15–37 % with by 6 years of follow-up [17–20]. In these early APBI trials, recurrence rates were even higher in some patient subsets, particularly those with invasive lobular cancer or extensive ductal carcinoma in situ. This stood in contrast to randomized studies with BCT using WB-XRT which demonstrated IBTR rates at 0.5–1 % per year. Interest in APBI waned with the publication of these recurrence rates.
Importantly, tissue side effects/toxicity was not a factor in any of early APBI trials with short- or long-term follow-up.
Subsequent review of these original APBI studies exposed major gaps in study design. There were: wide variations in patient selection; inconsistent surgical technique; and potential inaccuracies in radiation targeting. More specifically, many patients did not have preoperative mammography to assist in defining extent of disease or presence of satellite lesions. Often patients received gross “tumorectomies” of palpable disease rather than excision to tumor-free margins. Surgical pathology was incomplete or unavailable for some patients. For patients receiving either brachytherapy or external beam approaches, radiation treatments were targeted visually to the skin incision, rather than visualization of the cavity using ultrasound (US) or computed tomography (CT) guidance. In the single fraction IORT pilot study, patients with large tumors and multiple involved nodes were included. These are all scenarios that can contribute to high IBTR rates whether one follows lumpectomy with APBI or WB-XRT. To some extent these approaches represented a perspective that focused, high-dose radiation could, or would, effectively eliminate residual tumor foci more effectively compared with WB-XRT. This was not the case. Rather than a test of APBI, these early studies were a proving ground for the importance of patient selection, surgical technique, and radiation treatment planning with APBI.
Later APBI trials addressed these concerns [21, 22]. Phase I/II studies with modified APBI approaches have since demonstrated local recurrence rates comparable to those seen historically with WB-XRT [23]. Several professional societies, in particular the consensus guidelines from the American Society for radiation Oncology have since published guidelines for APBI that have refined patient selection, surgical technique, and radiation treatment planning: it is believed these criteria can enable patients to achieve IBTR rates with APBI comparable to those seen with conventional 6-week WB-XRT [24].
Patient Selection in APBI
There are multiple patient selection criteria to be considered (Table 18.4).
Factor | Criterion |
|---|---|
Patient factors | |
Age | >60 year |
BRCA1/2 mutation | Not present |
Pathologic factors | |
Tumor size | ≤2 cma |
T stage | T1 |
Margins | Negative by at least 2 mm |
Grade | Any |
LVSI | Nob |
ER status | Positive |
Multicentricity | Unicentric only |
Multifocality | Clinically unifocal with total size |
≤2.0 cmc | |
Histology | |
Invasive ductal or other favorable subtypesd | |
Pure DCIS | Not allowed |
EIC | Not allowed |
Associated LCIS | Allowed |
Nodal factors | |
N stage | pN0 (i−, i+) |
Nodal surgery | SN Bx or ALNDe |
Treatment factors | |
Neoadjuvant therapy | Not allowed |
Age: Local recurrence rates following BCS decrease with age [25]. The biological reasons have not been fully elucidated. Among current APBI trials, some investigators have duly set the lower age cut-off at 40. Others suggest that only post-menopausal candidates are appropriate. Only the ongoing, randomized NSABP B-39 trial permits enrollment of women under age 40: in this NSABP study, women 19 or older may be considered candidates [26]. Some have suggested there is a point at which radiation may not be necessary at all: clinical data suggests that women >age 70 with T1, endocrine sensitive tumors may experience such a small increase in local recurrence without radiation that BCS and tamoxifen alone are adequate [27]. In this CALBG study, at 10 years follow-up, 4 % of patients had succumbed to breast cancer related causes while 28 % died of other causes: 1 % of patients who had lumpectomy and WB-XRT then tamoxifen experienced an IBTR, while 10 % of patients with lumpectomy and tamoxifen alone experienced an IBTR. While this trial’s results are used as a guide in discussing treatment options with patients, most physicians take into account the overall health and potential longevity of the patient before making a no radiation/radiation recommendation: most specialists are still recommending post-lumpectomy radiation for patients over age 70 who are in excellent health. ASTRO consensus guidelines consider women ≥age 50 as “cautionary” candidates (Table 18.5), while women above age 60 are considered “suitable” for APBI.
Table 18.5
“Cautionary” group: any of these criteria should invoke caution and concern when considering APBI (from Smith et al. [24])
Factor | Criterion |
|---|---|
Patient factors | |
Age | 50–59 year |
Pathologic factors | |
Tumor size | 2.1–3.0 cma |
T stage | T0 or T2 |
Margins | Close (<2 mm) |
LVSI | Limited/focal |
ER status | Negativeb |
Multifocality | Clinically unifocal with total size 2.1–3.0 cmc |
Histology | Invasive lobular |
Pure DCIS | ≤3 cm |
EIC | ≤3 cm |
Preoperative workup: All APBI candidates should undergo conventional workup with physical exam, diagnostic mammography, and ultrasound as indicated. Breast magnetic resonance imaging (MRI) is considered by some to be a valuable adjunct in identifying prospective APBI candidates [28–30]. The goal of MRI in APBI candidates is to better define extent of disease, identify occult multifocal or multicentric lesions, and optimize chances for single-stage surgical resection. Fewer excisions will produce a smaller surgical cavity and a smaller radiation target volume regardless of which APBI approach is used. The benefits of single-stage excision are most critical for patients receiving APBI with single fraction IORT as one of the key benefits of this approach is the concept of 1 operation/1 radiation dose treatment: if a patient were to have close or positive margins, additional surgery and/or WB-XRT might be needed (with the IORT used as the boost dose). Suspicious lesions identified elsewhere in the breast by physical exam or imaging should be biopsied preoperatively with percutaneous techniques or excised at the time of lumpectomy for patients considering any of the 5-day forms of APBI. For patients considering single fraction IORT, suspicious satellite lesions should be sampled before surgery. APBI is generally contraindicated for almost all patients with multifocal disease and all patients with multicentric disease due to the potential for further satellite lesions and out of concern for such patients having a tendency to be at higher risk for local recurrence. The only APBI trial openly permitting limited multifocal disease is the ongoing NSABP B-39 trial.
Tumor size: Most clinical trials have utilized upper limits of tumor size of 2–3 cm. Multiple studies have demonstrated that large tumor size is associated with increased trends towards local recurrence. Furthermore, increasing tumor size leads to larger lumpectomy cavities which are problematic for all forms of APBI as larger tumor size requires larger volume excisions, which in turn leads to larger target volumes: as target volume increases, so does tissue toxicity due to the higher doses used with APBI. Such toxicity may manifest as adverse cosmetic results, fat necrosis, pain, etc. At this time, patients with locally advanced tumors are not considered optimal candidates for APBI even with a pCR. Efforts have been made to identify tumors that are so small that no radiation is necessary: to date, these studies have failed to identify a group of patients that does not receive significant benefit from post-lumpectomy radiation [31].
Surgical margins: All breast specimens and nodes in patients considering APBI should be evaluated by pathologists with considerable experience with breast disease. Tumor-free margins are critical: “tumorectomies” are unacceptable. An “optimal” tumor free margin has not been determined [32]. Most studies require or at least advise a tumor-free margin of 2 mm, while the NSABP B-39 study follows the traditional NSABP “no tumor on ink” paradigm.
Nodal status: Lymph node involvement has historically been associated with trends towards increased IBTR rates. Nodal involvement is considered by many a contraindication for APBI and has been deemed “unsuitable” by ASTRO criteria (Table 18.6). Institutional and registry data are conflicting [33, 34]. The NSABP B-39 trial permits enrollment of women with up to three involved nodes.
Table 18.6
Patients “unsuitable” for APBI outside of a clinical trial if any of these criteria are present (from Smith et al. [24])
Factor | Criterion |
|---|---|
Patient factors | |
Age | <50 year |
BRCA1/2 mutation | Present |
Pathologic factors | |
Tumor sizea | >3 cm |
T stage | T3-4 |
Margins | Positive |
LVSI | Extensive |
Multicentricity | Present |
Multifocality | If microscopically multifocal >3 cm in total size or if clinically multifocal |
Pure DCIS | If >3 cm in size |
EIC | If >3 cm in size |
Nodal factors | |
N stage | pN1, pN2, pN3 |
Nodal surgery | None performed |
Treatment factors | |
Neoadjuvant therapy | If used |
Tumor histology: Most APBI studies have focused on patients with pure invasive ductal cancer (IDC) or IDC mixed with limited ductal carcinoma in situ (DCIS). Extensive intraductal components (EIC) and invasive lobular cancer are considered a high risk feature for local recurrence and suboptimal for APBI [18]. Few studies have enrolled substantial numbers of patients with pure DCIS or invasive lobular cancer. Nonetheless, there is also conflicting institutional and registry data on the relative importance of these tumor factors as well [35, 36].
Tumor biology: Molecular profiling using intrinsic subset criteria (luminal A/B, basal, and so forth.) has emerged as a potentially meaningful factor in local recurrence, and hence has become a topic of interest with respect to patient selection for APBI. Further work needs to be done to understand whether profiling could lead to omission of post-surgical radiation or help identify optimal candidates [37]. Patients with luminal A tumors appear to have very low local recurrence rates and represent an optimal cohort for APBI [38]. Of note, most “profiling” studies to date have used immunohistochemistry surrogates rather than true gene expression profiling analyses to categorize tumor phenotype: there is a 10–15 % discordance between tumor profiling with IHC vs. tumor profiling through gene expression analysis [39].
Results from ongoing Phase II and especially Phase III studies should shed further light on the importance of each of these variables in patient selection.
Surgical Technique in APBI
Role of the surgeon: Surgeons play a critical role in APBI [40]. Often the surgeon is the first specialist to introduce the concept to potential candidates, educating patients regarding treatment options, and helping match each patient with an optimal treatment plan, whether it be BCS with conventional WB-XRT, BCS with APBI, BCS alone, neoadjuvant chemotherapy, or mastectomy. Patients considering APBI are ideally identified prior to resection of the tumor. This will ensure a thorough preoperative evaluation, enhance collaborative planning with colleagues in radiology and radiation oncology, and optimize chances of single-stage resection with tumor-free margins. For potential APBI candidates pre-surgical consultation with the treating radiation oncologist is ideal.
Tumor localization: Should a patient decide to pursue APBI, any and all localization tools should be used to maximize chances for single stage resection to tumor free margins and to minimize resection volume. This is accomplished, as possible, with the potential use of guidewires (X-ray, ultrasound, or MRI guided localization), radioactive seeds (ROLL technique), skin markers, and/or intraoperative ultrasound. Surgical specimens should always be oriented. This may not only facilitate efforts at re-excision, but may also guide the radiation oncologist to “high-risk” cavity margins. This author prefers resection of the specimen as a single piece of tissue rather than routine use of shaved margins. Should inspection, palpation, or imaging of the surgical specimen suggest a close margin, however, additional shave margins are taken as needed to help avoid a return to the OR for re-excision. Rarely, frozen section is performed intraoperatively to assess specimen edges. Ideally the index lesion is located centrally within the resection specimen such that the subsequent irradiation of cavity margins leads to symmetric coverage of tissue “at risk.”
Location of skin incision: Placement of the skin incision over the lesion may help guide post-surgical radiation with most forms of APBI by more clearly identifying the lumpectomy cavity vs. surrounding edema. Location of the incision over tumor is more critical for APBI to facilitate single-stage excision and accurate targeting of radiation.
Clip placement: With most forms of APBI intraoperative clip placement at lumpectomy cavity margins prior to skin closure can be of great benefit for identifying the cavity on subsequent CT scans [41, 42]. However, clips should be avoided when balloon devices are to be used as the clips can lead to balloon rupture. Clips are not necessary for single fraction IORT as the cavity margins are identified by direct visualization by the surgeon in the operating room.
Use of sentinel node biopsy: Sentinel node biopsy (SNB) should be performed in all women with invasive breast cancer considering APBI in order to stage the axilla (see patient selection, above). Nodal involvement is associated with slightly higher IBTR rates and falls in ASTRO’s “unsuitable” category.
APBI and ACOSOG Z0011: The ACOSOG Z-0011 study demonstrated that women who underwent lumpectomy and SNB for T1 or T2 invasive breast cancer and had 2 or fewer involved sentinel nodes did not benefit from a completion axillary node dissection (cALND) providing they subsequently received appropriate systemic therapy and WB-XRT [43]. The ACOSOG Z-0011 study did not enroll women who received lumpectomy and APBI. This study’s findings therefore are not directly applicable to patients considering APBI. As noted above, ASTRO guidelines consider any nodal involvement “unsuitable” for APBI.
The successful implementation of APBI relies on close cooperation between breast imaging, the surgical team, pathologists, and radiation oncologists. Poorly selected patients, poorly executed excisions, unnecessarily large or irregular surgical cavities, improper device placement, or poor radiation treatment planning can all undermine long-term treatment success.
The following section reviews the specific methods for delivering APBI. Each approach has slightly different technical requirements from the surgical and radiation oncology teams.
APBI Techniques
Overview
In general, there is no APBI technique that is optimal for any given patient. Part of the art of APBI is identifying patients appropriately and following that which approach is best for the patient at hand.
Brachytherapy
The first successful APBI implementation relied on interstitial brachytherapy [44]. Accurate in delivery of radiation, but technically challenging, interstitial brachytherapy techniques rely on the placement of a series of catheters placed in parallel through-and-through the breast around the surgical cavity. Low-dose-rate (LDR) sources were used initially, while interstitial brachytherapy teams now utilizes high-dose-rate (HDR) seeds delivered and distributed between and among the catheters with an HDR afterloading machine.
Successful interstitial brachytherapy paved the way for the development of intracavitary brachytherapy devices: rather than 10–20 catheters placed around the surgical cavity as with interstitial brachytherapy, intracavitary devices are usually a single catheter designed to lay within the surgical cavity. Device placement is faster, more comfortable, and can be accomplished any breast surgeon, rather than depending on access to the far more limited number of radiation oncologists who have training and experience with interstitial breast brachytherapy. The MammoSite was the first intracavitary device: it received FDA approval in 2005. This balloon device has a single channel through which the radioactive source can be delivered: single channel systems permit several dwell positions, but is quite limited in specifying dose distribution compared with the hundreds of dwell positions possible with interstitial brachytherapy. As the number of dwell positions increases, the radiation oncologist is better able to target cavity margins and avoid toxicity to skin and deep structures, such as muscle and rib. To address this concern, multichannel intracavity devices were developed. Some multichannel devices are balloon based, others not. Multichannel, intracavitary devices allow for greater control over radiation delivery with the convenience of a single skin entry point.
Most brachytherapy approaches utilize a treatment schedule of 34 Gy given in divided doses of 3.4 Gy BID over 5 days with a minimum of 6 h between treatments.
3D Conformal Radiotherapy
3D-CRT utilizes multiple external beams typically delivered via a linear accelerator that converge on the limited target volume around the lumpectomy cavity. 3D-CRT can be combined with other techniques, such as breathing synchronization, to further minimize target volume and dose to normal tissue. Variations on APBI with 3D-CRT include APBI with intensity modulated radiotherapy (IMRT) and APBI using protons.
Most 3D-CRT approaches utilize a treatment schedule of 38.5 Gy given in divided doses of 3.85 Gy BID over 5 days with a minimum of 6 h between treatments.
IORT
IORT delivers the entire therapeutic radiation treatment in a single dose through the open surgical incision while the patient is under anesthesia in the operating room. There are two major approaches to delivering single-fraction breast IORT. The ELIOT approach uses a collimator placed at the opening of the skin incision to direct high energy electrons to the target volume of cavity margins. The TARGIT approach relies on placement of a low energy brachytherapy sphere within the surgical cavity.
The single radiation dose for IORT is typically 20–21 Gy.
Detailed Review of APBI Techniques
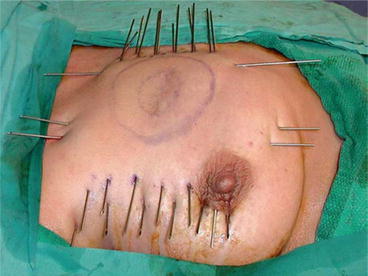
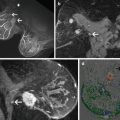
APBI with Interstitial Brachytherapy
APBI with interstitial brachytherapy is accomplished via a series of catheters placed in parallel which pass through the surgical cavity and surrounding breast tissue (Fig. 18.1). The catheters may be placed at the time of surgery, or as a separate invasive procedure postoperatively. Approximately 1 dozen catheters or more are placed into the breast tissue in two to three parallel rows using a free-hand approach or with a template “guide.” A CT scan is then performed to determine the relative position of the catheters in relation to the surgical cavity. Simulation software is used to determine dwell positions and durations for the Iridiium-192 seed in order to deliver the appropriate radiation dose to cavity margins while minimizing toxicity to surrounding tissue. In combination with special software, the dwell time and sequential positioning of the radioactive seed is controlled by a device known as an HDR afterloader (Fig. 18.2). Although the area around each dwell position is a “hot spot” at risk for developing toxicity, one of the great benefits of interstitial brachytherapy is that several hundred dwell positions are utilized as the seed can be placed at a series of locations in each of many catheters. The target volume for radiation delivery is 1.5 cm around the lumpectomy cavity, with restrictions placed on radiation delivery to skin and chest wall. The most commonly used treatment plan delivers a total of 34 Gy in 3.4 Gy fractions delivered twice daily over 5 days. After treatment is complete, catheters are removed. Despite the somewhat disturbing appearance of the catheters while they are in place, there is remarkably little evidence of the catheters in long-term follow-up. Phase I/II studies have validated this approach as being safe and yielding comparable results to historical controls for WB-XRT [45, 46].