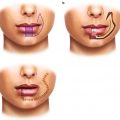
Primary
Neck
Definitive
Adjuvant
Definitive
Adjuvant
Cosmesis
Positive margins
Neck dissection not performed and:
Multiple lymph nodes
Preserve function
>5 mm depth
T3/T4 tumors or >2 cm in size
Extracapsular extension (with chemotherapy)
Medical comorbidities
Perineural invasion
>5 mm depth
Patient preference
Lymphovascular space invasion
Perineural invasion
Poor performance status
Recurrent tumors
Tumor grade
Unresectable (and chemotherapy if advanced)
Consider for:
Commissure involvement
Size > 2 cm
Clinically N-positive
Close margins
In deciding the primary treatment for lip cancers, one should consider the functional status of the patient, tumor size, tumor stage and histology, location of tumor on the lip, and cosmetic outcomes. Compared to radiotherapy, surgery is preferred for younger, healthier patients with smaller tumors that are at sufficient distant from the oral commissure. By contrast, definitive radiotherapy is a viable alternative for patients with poor performance status or medical comorbidities who cannot tolerate the anesthesia or surgical recovery. Still, especially for older patients, one must weigh these benefits with the hassle of multiple radiation therapy visits. Radiotherapy is also beneficial for tumors where surgical incisions may negatively impact the cosmetic outcomes. In these cases, radiotherapy provides better functional outcomes than surgery including improved sensation, lip elasticity, and intercommissural distance [42]. In addition, radiotherapy is often preferred for tumors involving the oral commissure as resection of these lesions may cause functional loss and/or poor cosmesis. Furthermore, radiotherapy remains the only local therapy for the rare case of unresectable disease. Finally, radiotherapy and surgery are likely equivalent for squamous cell carcinomas and basal cell carcinomas, but surgery is indicated for rare cases of sarcomas, adenocarcinomas, melanomas, and Merkel cell tumors that are likely more resistant to radiotherapy. Thus, radiotherapy is often used for SCCs or BCCs occurring in older patients, those with poor functional status, or in cases where surgery would result in poor functional or cosmetic outcomes.
Since wide local excision provides pathological staging and a more accurate assessment for tumor aggressiveness, several pathological features also indicate the use of adjuvant radiotherapy. Adjuvant radiotherapy to the surgical bed is considered for recurrent tumors, positive margins, perineural invasion, tumor thickness greater than 5 mm, or lymphovascular invasion. de Visscher et al. has reported that lip cancers after surgery have a local recurrence of greater than 10 % for positive margins (38 % chance for recurrence), thickness >5 mm (13 % chance for recurrence), tumor size greater than 1.5 cm (33 % chance for recurrence), or perineural invasion (20 % chance for recurrence) [13]. These risk factors mimic the indications for postoperative radiotherapy for SCC in other mucosal sites of the head and neck [7, 24, 26, 28, 41]. In addition, Rowe et al. showed that recurrent SCC of the lip locally recurs after re-excision in 23 % of patients [37]. One unknown question remains: how close must the cancer be to the surgical margin to indicate need for adjuvant radiotherapy? For mucosal SCCs of the head and neck, margins of 5 mm or less predispose to increased recurrence [28]. However, since SCC of the lip behave more like cutaneous SCCs, closer surgical margins are likely acceptable. Therefore, adjuvant radiotherapy for close margins or dysplasia at the margins is at the discretion of the treating physician, but ultimately adjuvant therapy may be avoided. Similarly, tumors involving the bone may benefit from a combined approach of surgery and radiotherapy, as these are rare but more aggressive tumors. Thus, adjuvant radiotherapy is often used for positive margins, perineural invasion, and larger and/or thicker tumors.
The decision to treat the neck is a complicated and debated topic. The presence of involved lymph nodes portends a dramatically worse prognosis where 5-year overall survivals of almost 100 % without lymph node involvement drop to 50–80 % with lymph node involvement [13, 44, 48]. Overall, early-stage tumors have less than 10 % risk of lymph node involvement [44]. Depending on risk factors, 5–30 % of patients with clinically negative lymph nodes will have pathological evidence of disease [13, 45]. Conversely, approximately 50 % of patients with clinically positive lymph nodes were pathologically negative [44].
The main risk factors for microscopic lymph node positivity in a clinically N0 neck include advanced tumors, commissure involvement, tumor grade, tumor size, increased tumor depth, and perineural invasion. Vartanian et al. showed that 27 % of T3/4 tumors have occult lymph node spread compared to 7 % of T1/2 tumors [45]. Furthermore, 22 % of patients with oral commissure involvement harbored occult diseased in the neck compared to 5 % without commissure involvement. In addition, patients with perineural invasion, recurrent tumors, thickness >5 mm, or tumor diameters >2 cm have a greater than 10 % risk of lymph node involvement at presentation or relapse [13, 46, 48]. After a neck dissection, patients also likely benefit from adjuvant radiotherapy where the neck dissection shows intermediate or high-risk features. Extrapolating from mucosal SCC of the head and neck, patients with multiple positive lymph nodes benefit from adjuvant radiotherapy [23, 40, 41] while lymph nodes showing extracapsular extension require adjuvant chemoradiation to the neck [5, 6, 10]. Therefore, patients should have the neck addressed with surgery or radiotherapy in cases with clinically palpable lymph nodes or with clinically negative lymph nodes but tumors having oral commissure involvement, perineural invasion, thickness greater than 5 mm, or stage T3/T4. In the cases of neck dissection, patients with multiple positive lymph nodes or extracapsular extension need adjuvant radiation or chemoradiation, respectively.
Similar to other mucosal head and neck cancers, lip cancers have a predictable route of lymph node spread [44, 48]. In general, the submental and submaxillary lymph nodes (level Ia and Ib) were most commonly involved with 66 % of positive lymph nodes detected in neck dissections. The second echelon of spread is the upper jugular (level II; 19 % of positive lymph nodes) and mid jugular chain (level III; 12 % of positive lymph nodes). Cancers rarely spread to the lower jugular chain (level IV lymph nodes) and all occurred in the setting of positive lymph nodes in other levels. For upper lip cancers, the parotid bed may also be clinically at risk. Therefore, one should consider addressing lymph node levels I–III in clinically N0 patients with high-risk features and additional lymph node levels when clinically indicated.
In summary, definitive radiotherapy is indicated for patients with SCCs or BCCs of the lip with poor performance status, medical comorbidities or tumors where surgery results in unacceptable cosmetic or functional outcomes. Adjuvant radiotherapy is indicated for perineural invasion, positive margins, and tumors larger than 2 cm or thicker than 5 mm. Unless a neck dissection is performed, the regional lymphatics require radiotherapy if the primary tumor has perineural invasion, oral commissure involvement, T3/T4, or thickness >5 mm. Finally, adjuvant radiotherapy is given to the neck with multiple positive lymph nodes or adjuvant chemoradiation when extracapsular extension is present.
6.3 Radiation Therapy Versus Surgery
Given the rarity of this tumor, there are no and likely will never be any randomized trials to address the equivalency of radiotherapy and surgery. The comparison between surgery and radiotherapy is limited to small retrospective reviews that indicate nearly equivalent outcomes where radiotherapy provides as good as or better cosmesis and functional outcomes. Still, any conclusion regarding control rates are taken with a grain of salt as radiotherapy is more often given to patients with clinically staged cancers that may underestimate the true extent of disease than that obtained by pathological staging with surgery. Furthermore, surgery is often performed on younger people with better performance status that may also result in improved control rates as well as functional and cosmetic outcomes. Therefore, radiotherapy outcomes are likely equivalent to surgery even though patients undergoing radiotherapy likely have more extensive tumors and/or have increased comorbidities.
Table 6.2 details the results of several retrospective series reporting the outcomes of surgery and/or radiotherapy for lip SCCs [2, 8, 11, 13, 14, 19, 30, 35, 39, 42, 43, 46]. Only four reports directly compared their experience with surgery and radiotherapy. de Visscher et al. reported on 256 patients with stage I lip cancers who underwent surgery (n = 166) or radiotherapy (n = 90) [14]. While the 5-year overall survival was 74 % and did not differ between modalities, disease-free survival was significantly better with surgery than radiotherapy (p = 0.04). However, this difference in DFS was associated with higher nodal failure rates where regional failure occurred in 4.8 % with surgery compared to 12.2 % with radiotherapy. By contrast, there was no difference in local failure rates with 3.6 % local failure with surgery compared to 4.4 % local failure with radiotherapy. These differences again likely reflected the biases in patient selection as the radiotherapy cohort had larger tumors, were more poorly differentiated and the surgery arm had shorter follow-up. Similarly, a study by Luna-Ortiz showed no differences in local failure for upper lip cancers when treated by surgery (53 % DFS at 5 years) or radiotherapy (69 % DFS at 5 years) albeit with much lower than expected control rates [30]. By contrast, a much older study by Ashley et al. compared 106 patients treated with surgery and 43 patients treated with radiotherapy [2]. The 10-year DFS was 87 % with surgery and 77 % with radiotherapy. Of note, eight of nine surgical patients failed in the neck while five out ten radiotherapy patients failed in the neck. By contrast, local failure occurred in one of nine surgical patients compared to five out of ten radiotherapy patients. These differences in local failure by de Visscher et al. and Ashley et al. may be explained by differences in the eras of radiotherapy planning and lower radiotherapy doses where doses ranged from 35 to 60 Gy in the study by Ashley et al. and 48–60 Gy in the study by de Visscher et al. Finally, Stanc et al. reported on the functional outcomes on 37 patients treated with surgery or radiotherapy [42]. Patients treated with radiotherapy had essentially normal lip function. Although several different surgical techniques were used, surgical-treated patients had significantly worse sensation, intercommissural distance, and lip elasticity. Furthermore, surgical patients had worse oral continence compared to radiotherapy (42 % vs. 11 %).
Table 6.2
Outcomes of lip cancers treated with radiotherapy or surgery
Study | Technique | Patients | LC | RC | DFS | OS | SE |
|---|---|---|---|---|---|---|---|
Stranc et al. [42] | Surgery | 19 | N.S. | N.S. | N.S. | N.S. | Oral incontinence 8/19, Difficulty eating 3/19 |
EBRT (44.25 Gy/11 fxn) | 18 | N.S. | N.S. | N.S. | N.S. | Oral incontinence 2/18, Difficulty eating 0/18 | |
De Visscher et al. [14] (All T1) | Surgery | 166 | 3 % | 5 % | 5y = 90 % | 5y = 81 % | N.S. |
10y = 90 % | 10y = 64 % | ||||||
EBRT (48–60 Gy) | 90 | 4 % | 12 % | 5y = 84 %. | 5y = 74 % | N.S. | |
10y =81 % | 10y = 74 % | ||||||
Ashley et al. [2] | Surgery | 106 | 99 % | 92 % | 5y = 87 % | 5y = 90 % | N.S. |
EBRT (35–60 Gy) | 43 | 88 % | 88 % | 5y = 77 % | 5y = 91 % | N.S. | |
Luna-Ortiz et al. [30] (upper lip only) | Surgery | 17 | N.S. | N.S. | 53 % | N.S. | N.S. |
EBRT | 29 | N.S | N.S | 69 % | N.S. | N.S. | |
Gooris et al. [19] | EBRT (54–56 Gy) | T1 = 37 | T1 = 100 % | T1 = 97 % | N.S. |  97 % 97 % | 16 % oral incontinence, 53 % dryness, 30 % numbness |
Surgery - > EBRT | T2 = 15 | T2 = 80 % | T2 = 100 % | N.S. | |||
T3 = 2 | T3 = 50 % | T3 = 100 % | 67 % dryness, 33 % pain | ||||
16 | 100 % | 100 % | |||||
De Visscher et al. [11] | EBRT (3 with BRT) | T1 = 89 | T1 = 98 % | T1 = 88 % | 5y = 66 % | 5y = 74 % | No soft tissue or bone necrosis. |
T2 = 14 | T2 = 77 % | T2 = 93 % | 10y = 59 % | 10y = 74 % | |||
T3 = 2 | T3 = 100 % | ||||||
Cerezo et al. [8] | EBRT (18–52 Gy) | T1 = 86 | T1 = 96 % | T1 = 96 % | N.S. | 5y = 81 % | 5 % with late ulceration |
T2/3 = 14 | T2/T3 = 94 % | T2/3 = 80 | 10y = 71 % | ||||
Petrovich et al. [33] | EBRT (30–48 Gy) | T1N0 = 173 | N.S. | N.S. | T1 = 94 % | N.S. | N.S. |
T2N0 = 48 | T2 = 94 % | ||||||
T3/N1 =10 | T3 or N1 = 90 % | ||||||
T4/N2–3 =19 | T4 or N2/3 = 53 % | ||||||
Sykes et al. [43] | EBRT (40 Gy) | 26 | 92 % | 96 % | N.S. | N.S. | 88 % (soft tissue necrosis, persistent soreness) |
de Visscher et al. [13] | Surgery | T1 = 170 | 95 % | 95 % | 95 % | 5y = 78 % | N.S. |
T2 = 9 | 10y = 61 % | ||||||
Salgarelli et al. [39] | Surgery | T1 = 76 | N.S. | N.S. | 97 % | 90 % | N.S. |
T2 = 22 | |||||||
T3 = 8 | |||||||
Vukadinovic et al. [46] | Surgery | 223 | 89 % | 95 % | N.S. | N.S. | N.S. |
In summary, surgery and radiotherapy likely provide equivalent overall survival and local control. Any differences in local control previously reported in the literature are likely due to differences in patient selection and older radiotherapy techniques. In our experience, radiotherapy for older patients or for tumors that are larger or involve the oral commissure provides equivalent tumor control and better cosmesis and functional outcomes compared to surgery.
6.4 Radiotherapy Techniques for Treating the Lip
6.4.1 External Beam Radiotherapy
EBRT, also referred to as teletherapy, refers to radiation given at a distance from the patient. In the past, EBRT was delivered with orthovoltage photons with energies ranging from 150 to 250 kVp that was sufficient to cover the tumor depth but also of low-enough energies so that normal adjacent tissues could be shielded and protected from radiotherapy. With the advent of megavoltage linear accelerators, electron therapy has become a mainstay of treatment for lip cancers.
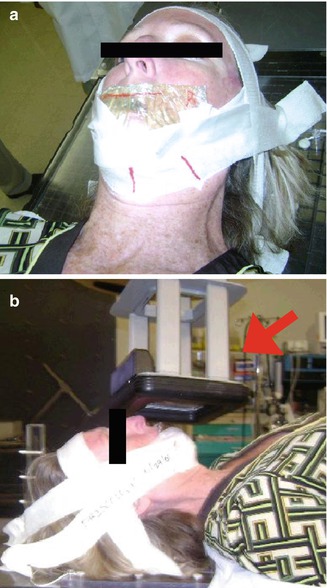
The physical properties of electrons, which possess mass, and photons, which do not possess mass, must be accounted for in the radiotherapy planning. For field setup, orthovoltage photons require a 1 cm margin on the tumor while megavoltage electrons require a 1.5 cm margin due to the “bowing in” of the higher isodose lines in an electron beam. Furthermore, with electron beam therapy, the lower isodoses “bow outward” and, therefore, electrons deposit lower radiation doses outside of the treatment field. This “bowing outward” must be taken into account for overlapping or adjacent electron fields. In addition, electrons reach maximum dose after a few millimeters of tissue and therefore require a 0.5–1 cm bolus in order to treat the superficial extent of the tumor adequately. This bolus is not needed in orthovoltage treatment as the skin surface receives full dose. Finally, shielding of normal tissues is different for electrons and orthovoltage photons. For orthovoltage photons, normal tissues can be blocked with 2 mm lead shields placed in the gingival-buccal sulcus behind the mucosal surface of the lip. This lead shield protects the alveolar ridge and mandible. By contrast, megavoltage electrons require slightly thicker lead shields of 3–5 mm in order to attenuate transmission. In addition, a small amount of wax is placed on the mucosal surface of the shield. This wax absorbs electrons that are reflected back (“backscattered electrons”) to the mucosal surface and otherwise would increase hot spots and, consequently, mucositis.
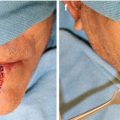
The planning of the orthovoltage photons and electrons are also different. Orthovoltage photons are prescribed with photon filter, thickness of flattening filter, and the source to skin distance (SSD). For example, orthovoltage treatments cover a superficial tumor usually using a 200–300 kVp photons with a 2 mm Al flattening filter at a 20 cm SSD. By contrast, electron therapy is prescribed using energies and to an isodose line; usually, 6–9 MeV electrons are prescribed to the 90 % isodose line. In addition, the diameter of the electron field has to be at least one-half of the energy in order to achieve appropriate dose buildup at the desired depth. Therefore, for a 6 MeV electron, the field diameter must be at least 3 cm. Finally, the differences in relative biological effectiveness of orthovoltage photons and electrons require electrons to receive approximately 10 % higher total dose. For orthovoltage photons, the tumor usually receives 48–51 Gy in 2.5–3 Gy fractions. For electrons, the tumor is treated to 51–54 Gy in similar fraction sizes. For tumors abutting cartilage or bone, 60 Gy is given in 2 Gy fractions to minimize the risks of necrosis. Therefore, differences in the physical properties of orthovoltage and electron beam therapies require slightly different variations in treatment technique in order to treat the tumor adequately. Figure 6.1 shows an example of a patient treated with megavoltage electrons. Table 6.3 compares the characteristics needed to prescribe orthovoltage photon or electron beam therapy.