Classic randomized trials documented the benefit of postmastectomy radiotherapy in women with node-positive or locally advanced breast cancer. Modern advances in surgical therapy, systemic therapy, and radiotherapy, however, along with an improved understanding of cancer biology, have called into question previously assumed recurrence risks and treatment benefits. This article explores the impact of tumor biology and genomic medicine on utilization of postmastectomy radiotherapy and how treatment decision making is moving beyond TNM-based predictors.
Key points
- •
Breast cancer biologic subtype is an important predictor of locoregional recurrence (LRR) and should be considered, along with tumor–node–metastasis (TNM) parameters, in the determination of benefit from postmastectomy radiotherapy (PMRT).
- •
The biology of node-negative tumors greater than or equal to 5 cm is heterogeneous. Tumor size alone is likely insufficient to estimate degree of benefit from PMRT.
- •
LRR risk in early-stage patients after mastectomy is generally low. Patients with multiple risk factors, however, in particular those with triple-negative tumors, may derive benefit from PMRT. Clinical trials are needed to establish this benefit.
- •
Early data link gene expression to risk of LRR and demonstrate promise as a future predictive tool.
- •
The role of chemotherapy response biomarkers in predicting LRR is incompletely understood. Until additional data are available, a combination of clinical and pathologic staging parameters should be considered.
Introduction
PMRT has long been a component of comprehensive breast cancer management, particularly in the setting of locally advanced disease. Historic trials from Denmark and British Columbia enrolled a large number of primarily node-positive patients to evaluate the use of PMRT in a randomized fashion. Both local control and overall survival (OS) rates were significantly improved with the use of radiotherapy (RT). As described extensively in the past, however, these trials had significant problems that limit their applicability to present-day practice. In particular, modern nodal staging and imaging techniques have resulted in significant stage migration. In combination with advances in cytotoxic chemotherapy and endocrine therapy as well as the advent of targeted therapy, extrapolating the extent of recurrence risk from these landmark trials is difficult.
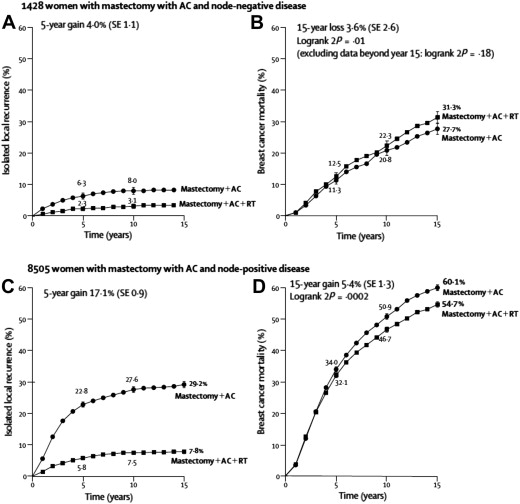
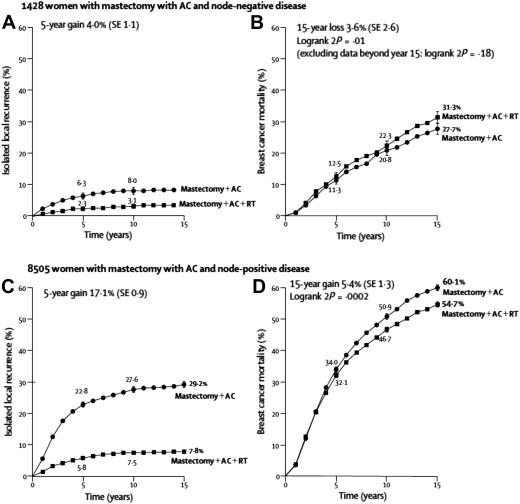
Many subsequent series have suggested that the modern risk of LRR is substantially lower than previously reported in both breast-conserving and postmastectomy settings. This is particularly relevant in the ongoing effort to maintain a desirable benefit/risk ratio such that patients obtain maximum tumor control at a minimum cost. RT, however, is also radically different from that used in the classic trials and evidence suggests that morbidity is decreasing. The 2005 update of the Early Breast Cancer Trialists’ Collaborative Group meta-analysis revealed that the benefit in breast cancer survival attributable to the use of RT has translated into OS, in contrast to prior reports in which the breast cancer–specific survival benefit from RT was offset by a near-equal increase in treatment-related mortality. These data suggest that for every 4 local recurrences that are prevented, 1 life is saved. This supports a more generous use of RT when the locoregional risk is intermediate or high. Even within the context of this positive RT impact, however, the data also suggest that there is a point of diminishing returns (ie, when the local recurrence risk becomes sufficiently low, the benefit of therapy does not outweigh the risks) ( Fig. 1 ).
Therefore, radiation oncologists must carefully estimate a patient’s starting risk of LRR in order to ascertain the benefit of therapy. Classically, this risk has been defined largely by TNM parameters. In the past decade, however, understanding of breast cancer biology has radically changed. Tumor size and nodal involvement are now joined by biologic subtypes that drive prognosis and therapy. This section explores the impact of these changes on the prescription of locoregional RT.
Introduction
PMRT has long been a component of comprehensive breast cancer management, particularly in the setting of locally advanced disease. Historic trials from Denmark and British Columbia enrolled a large number of primarily node-positive patients to evaluate the use of PMRT in a randomized fashion. Both local control and overall survival (OS) rates were significantly improved with the use of radiotherapy (RT). As described extensively in the past, however, these trials had significant problems that limit their applicability to present-day practice. In particular, modern nodal staging and imaging techniques have resulted in significant stage migration. In combination with advances in cytotoxic chemotherapy and endocrine therapy as well as the advent of targeted therapy, extrapolating the extent of recurrence risk from these landmark trials is difficult.
Many subsequent series have suggested that the modern risk of LRR is substantially lower than previously reported in both breast-conserving and postmastectomy settings. This is particularly relevant in the ongoing effort to maintain a desirable benefit/risk ratio such that patients obtain maximum tumor control at a minimum cost. RT, however, is also radically different from that used in the classic trials and evidence suggests that morbidity is decreasing. The 2005 update of the Early Breast Cancer Trialists’ Collaborative Group meta-analysis revealed that the benefit in breast cancer survival attributable to the use of RT has translated into OS, in contrast to prior reports in which the breast cancer–specific survival benefit from RT was offset by a near-equal increase in treatment-related mortality. These data suggest that for every 4 local recurrences that are prevented, 1 life is saved. This supports a more generous use of RT when the locoregional risk is intermediate or high. Even within the context of this positive RT impact, however, the data also suggest that there is a point of diminishing returns (ie, when the local recurrence risk becomes sufficiently low, the benefit of therapy does not outweigh the risks) ( Fig. 1 ).
Therefore, radiation oncologists must carefully estimate a patient’s starting risk of LRR in order to ascertain the benefit of therapy. Classically, this risk has been defined largely by TNM parameters. In the past decade, however, understanding of breast cancer biology has radically changed. Tumor size and nodal involvement are now joined by biologic subtypes that drive prognosis and therapy. This section explores the impact of these changes on the prescription of locoregional RT.
Changing paradigm
In addition to advances in therapeutics, understanding of breast cancer as a disease has been transformed by work published in 2000 by Perou and colleagues. Several distinct subtypes of breast cancer were identified with unique patterns of gene expression. These have subsequently proved both prognostic and predictive. These subtypes have so significantly changed the approach that clinical trials evaluating systemic therapy are often now typically developed for a specific subtype. This has allowed further development of biomarkers, such as chemotherapy response, to be incorporated into routine practice. Although TMN staging remains a critical part of determining outcomes and need for therapy, tumor biology plays an equally important role.
Data have only recently started to emerge, however, on the impact of tumor biology on LRR risk. In 2008, Nguyen and colleagues published one of the first articles addressing the impact of the 4 main breast cancer subtypes on local recurrence after breast-conserving therapy (BCT). Both human epidermal growth factor receptor 2 (HER2)-positive and basal subtypes demonstrated a higher risk of recurrence than the more favorable luminal subtypes. Subsequently, multiple additional series have verified this finding in both postmastectomy and breast conservation scenarios. These series seem to suggest that the subtypes may be both prognostic in regards to risk of local recurrence and predictive in evaluating the benefit of RT. Kyndi and coworkers demonstrated that the subgroup of patients with steroid receptor negativity with or without HER2 positivity experienced smaller improvements in local control with the addition of radiation. As a result, the survival benefit attributable to radiation was only seen in the more favorable subgroups. These data predate, however, modern systemic and targeted therapy such that competing systemic risks likely mask any radiation benefit.
A similar effect can be seen in a more modern series evaluating approximately 3000 patients who underwent mastectomy (only 25% received RT) or BCT (all received RT). In patients treated with mastectomy, molecular subtype clearly predicted risk of local recurrence. These differences should largely have disappeared in the breast-conserving group, where all patients received RT, if all of the breast cancer subtypes were equally responsive to RT. Instead, a pattern of subtype-specific locoregional risk persists, suggesting that there are inherent differences in a priori risk as well as variability in radiation response among the breast cancer subtypes ( Fig. 2 ).
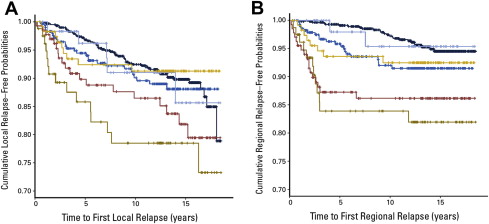
These data, in conjunction with what has been learned about the impact of biologic subtype on chemotherapy response, suggest that revisiting the approach to PMRT may be in order. The classic distinctions of nodal involvement and tumor size may no longer be sufficient, in isolation, to guide therapeutic decision making. Although it is unlikely that the classic trials will be repeated by specific biologic subtypes, at the least this biologic information needs to be incorporated into future radiation clinical trials. Patients with early-stage disease, previously considered ineligible for PMRT, may benefit at much earlier stages, and some patients with more advanced disease but favorable biology may not require adjuvant RT. In addition, high-risk patients with aggressive biology may require some form of radiosensitization in order to gain the full benefit of therapy whereas those with favorable biology may benefit from strategies designed to limit radiation toxicity.
Risk stratification in node-negative cancer
PMRT has not traditionally been offered for patients with node-negative disease, except in the case of T3/4 tumors or positive margins. A 2005 survey conducted in North America and Europe showed that 88.8% of American Radiation Oncologists and 84.8% of European Radiation Oncologists would offer PMRT to patients with T3N0 breast cancer. Data are available, however, that question both the automatic use of RT in the setting of T3 disease and the automatic exclusion of RT in the setting of T1/2N0 disease with certain biologic risk factors.
T3N0
The T3N0 subgroup of breast cancer patients is small, comprising approximately 1% of new breast cancer diagnoses, so there are few data sets to guide clinical practice. In support of adjuvant therapy, in node-negative patients enrolled in the Danish 82b and 82c postmastectomy radiation trials, PMRT resulted in a substantial reduction in risk of LRR—5% versus 22% in 82b, and 6% versus 23% in 82c. One caveat frequently noted regarding these trials, however, is that their overall LRR rates in all subsets are higher than in many modern series. Additionally, the median number of nodes in the axillary dissection was lower than is now considered standard, the systemic therapy was older, and T4 tumors were included. Nonetheless, there was a benefit to RT in this subgroup. Another small study evaluated 38 patients with T3N0 disease and found an overall LRR of 16% at 5 years—9% with RT and 60% without RT. Again this is a small study, as expected with such a limited patient group, so generalizations are difficult.
In contrast, a retrospective analysis of 313 patients with node-negative tumors greater than or equal to 5 cm enrolled in 5 National Surgical Adjuvant Breast and Bowel Project (NSABP) clinical trials, treated from 1981 to 1998 with mastectomy but without RT, noted a low incidence of locoregional failure of 7.1% at 10 years ; 74.5% of patients had some form of systemic therapy, and this seemed to decrease LRR risk, because the rate was 12.6% in patients who received no systemic therapy versus 4.6% to 5.6% in patients who received chemotherapy and/or tamoxifen; 24 of 28 recurrences were on the chest wall. Similarly, a multi-institutional retrospective series of patients with node-negative tumors greater than or equal to 5 cm treated from 1981 to 2002 identified 70 patients treated with mastectomy and adjuvant systemic therapy but no RT ; 57% of patients had some form of systemic therapy. The investigators noted a 5-year LRR rate of 7.6%, with 4 of 5 failures on the chest wall. Lymphovascular invasion (LVI) was significantly associated with LRR risk as well as with disease-free survival and OS in univariate and multivariate analysis.
Goulart and colleauges reported outcomes for 100 patients with node-negative tumors greater than or equal to 5 cm treated from 1989 to 2000 with mastectomy, with or without PMRT. The cumulative 10-year LRR was 2.3% with PMRT and 8.9% without PMRT. For the subset of patients with grade 3 histology and no PMRT, however, the LRR rate at 10 years was 17%; 4 of 6 recurrences were on the chest wall. Approximately 50% of patients had chemotherapy, and 50% had hormonal therapy. One factor that may limit applicability of these data is that all 3 of these studies were significantly weighted toward the lower end of the size spectrum, with 46%, 34%, and 28% of patients having 5.0 cm tumors, respectively. They highlight, however, a population of large tumors with low LRR rates after PMRT, particularly if systemic therapy is used.
Overall, the conflicting data suggest that the biology of tumors greater than 5 cm varies and that this is a heterogeneous population for which size alone is insufficient to determine LRR risk and benefit of PMRT. Biologically, tumors that grow to a large size without spreading to lymph nodes may in general be less aggressive. Features, such as high grade and LVI, however, may point to a subset with more aggressive biology likely to derive a greater benefit from PMRT. Additionally, PMRT should likely still be strongly considered for patients with tumors significantly larger than 5 cm, because this subset of patients is poorly represented in any data set. Finally, whether or not a patient is eligible for systemic therapy in the form of chemotherapy and/or hormonal therapy should be weighed, because this can mitigate LRR risk as well. In all the data sets, the majority of locoregional failures were confined to the chest wall, suggesting that this is the highest-risk target volume.
T1/2N0
T1/2N0 cancers treated with mastectomy have traditionally been thought of as low risk and, therefore, not warranting consideration of PMRT. A growing set of data question this, however, as an across-the-board approach for all patients. An analysis of women treated from 1981 to 1985, in a randomized trial with mastectomy without PMRT, identified factors associated with LRR in 1275 node-negative patients. Two-thirds of the women received 1 cycle of chemotherapy. In premenopausal women, tumor size greater than 2 cm and vascular invasion (VI) were associated with increased risk for LRR. The LRR rate with no risk factor was 6%, 13% with either factor alone, and 15% with both. In postmenopausal women, VI alone was associated with LRR, with an observed recurrence rate of 6% without VI versus 14% with VI.
Jagsi and colleagues identified 877 patients with T1–T3N0 tumors treated from 1980 to 2000 with mastectomy and axillary node dissection. No patients received PMRT, and T4 tumors were excluded. The cumulative incidence of LRR was 6%. Factors associated with increased LRR risk were tumor size greater than 2 cm, margin less than 2 mm, premenopausal status, and LVI. LRR was 1.2% with no risk factors, 10% with 1 risk factor, 17.9% with 2 risk factors, and 40.6% with 3 risk factors; 80% of failures were on the chest wall. An updated series from the same institution, with 1136 patients treated from 1980 to 2004, showed the association of tumor size greater than 2 cm, LVI, close or positive margin, age less than or equal to 50, and no systemic therapy with increased risk for LRR. LRR was 2.0% with no risk factors versus 19.7% with 3 or more factors. Again, close to 80% of failures were on the chest wall alone.
A series from British Columbia of 1505 women with T1/2N0 cancers treated from 1989 to 1999 with mastectomy and no adjuvant RT reported an overall LRR rate of 7.8%. Approximately half the women had systemic chemotherapy. Histologic grade, LVI, T stage, and systemic therapy use were identified as independent predictors of LRR. High-grade histology as a sole risk factor was associated with an LRR rate of 12.1% versus 5.5% for low grade or intermediate grade. Concomitant LVI increased LRR risk to 21.2%. High tumor grade plus size greater than 2 cm had an LRR of 13.4% with systemic therapy and 23.2% without systemic therapy. To evaluate this further, a subset of 763 women who all received adjuvant systemic therapy after mastectomy was selected from the cohort to evaluate relapse based on the presence or absence of LVI. They found that LVI was associated with significantly higher risk of LRR. If LVI was present in conjunction with age less than 50 years, premenopausal status, grade III histology, or estrogen receptor (ER)-negative disease, the LRR risk increased to 15% to 20%.
Building on these findings, a recent prospective randomized multicenter trial evaluating PMRT restricted eligibility to women with early-stage cancer but triple-negative biology—681 Chinese women were treated with mastectomy plus adjuvant chemotherapy versus chemotherapy plus radiation and 82% of patients had node-negative disease. At a median follow-up of 86.5 months, the recurrence rate with chemotherapy alone was 25.4% versus 11.7% with chemotherapy plus RT. Five-year OS was also significantly improved with the addition of RT, to 90.4% versus 78.7% with chemotherapy alone. Although the degree of radiation benefit likely reflects, in part, a higher-risk patient population (62% of patients were less than or equal to 50 years old, and 18% were node positive) and suboptimal chemotherapy (cyclophosphamide, methotrexate, and 5-fluorouracil), it provides compelling preliminary data for confirmatory clinical trials.
Taken as a whole, the data for T1/2N0 tumors show a low LRR rate when all patients are considered as a group. There are, however, biologic subgroups with significantly higher risk for LRR. A review of the literature and meta-analysis of randomized trials for node-negative tumors treated with mastectomy and no radiation found that LRR risk is increased with grade 3 histology, LVI, tumor size greater than 2 cm, close margins, and premenopausal status or age less than 50, all seen in greater proportions in biologically aggressive tumors. The baseline LRR risk with no risk features is uniformly close to 5% but ranges from 15% to 40% with 2 or more risk factors.
The meta-analysis (discussed previously) showed a large LRR and OS benefit for PMRT. The randomized trials included in the meta-analysis used older or no chemotherapy, lacked data regarding axillary node dissection, or had low median numbers of dissected nodes, and the N0 tumors were often T3. This result is in agreement, however, with the improvement in LRR (and OS) seen in the randomized Chinese trial of adjuvant RT for T1/2N0 triple-negative breast cancer (discussed previously), suggesting that there is a subgroup of node-negative patients who derive significant benefit from PMRT. A majority of LRR seen in all the node-negative data sets are confined to the chest wall, suggesting that treatment of the chest wall alone may be sufficient to mitigate the majority of the LRR risk in these patients.
The biologic factors associated with LRR in node-negative cancers may also be useful for risk stratification in patients with 1 to 3 involved nodes. Several retrospective series have identified features that predict increased LRR risk in patients with 1 to 3 nodes similar to those that predict LRR in node-negative patients, again supporting the idea that tumor size or nodal status alone is not sufficient to fully define risk. The ongoing Selective Use of Postoperative Radiotherapy after Mastectomy trial randomizes T1/2N0/1 and T3N0 patients with grade 3 histology or LVI to PMRT to the chest wall versus observation. The results of this trial will hopefully yield important information regarding the benefit of PMRT for patients with low-risk T/N stage but high-risk biology as well as the appropriateness of chest wall RT alone.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree