25 Radiosurgery
Stereotactic radiosurgery (SRS) is most often defined as the delivery of a single high dose of ionizing radiation to an intracranial target defined using stereotactic imaging.1 Although the definition has been expanded by some to include up to five fractions delivered using stereotactic technology, this chapter is limited to a discussion of the single-dose approach and results. Extracranial SRS is discussed in Chapter 75.
The use of accelerated particles for highly conformal treatment of brain tumors developed following the proposal of physicist Robert Wilson and coworkers at the University of California, Berkeley.2 The initial clinical trial using protons to treat pituitary disorders was begun in 1954 with 840 patients subsequently treated with protons or helium ions.3,4 These focal-radiation delivery methods were further developed and the term radiosurgery was coined by Swedish neurosurgeon Lars Leksell and radiobiologist Borje Larsson of the Karolinska Institute in Stockholm, initially for treatment of functional disorders.5 Several different delivery techniques result in a dose distribution that is highly conformal to the target. Dose fall-off outside the target is precipitous and therefore only the target and immediately adjacent normal tissue are included in the high-dose region. After more than 40 years of development, radiosurgery has entered the mainstream of medical care. Since the 1960s, more than 1,000,000 patients have been treated with some form of radiosurgery, most within the past 10 years. Radiosurgery is now practiced at more than 250 facilities in the United States.
In North America, patients who undergo radiosurgery are usually selected, treated, and followed by a dedicated multidisciplinary radiosurgery team of experts that includes radiation oncologists, neurosurgeons, physicists, nurses, and technologists; the same radiosurgery team provides quality assurance. This multidisciplinary approach to therapy has received support from the radiosurgery task forces of societies representing both neurosurgery and radiation oncology, and is considered the standard of care in the United States.1,6–8
Techniques
Stereotaxis
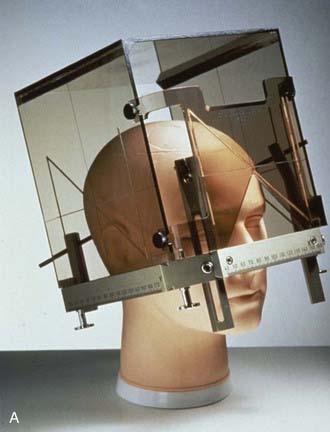
The term stereotactic (from the Greek stereo and taxis, ordered in three dimensions) refers to techniques in which intracranial targets are localized precisely in three-dimensional space for introduction of a probe for biopsy or for delivery of localized radiotherapy. The basis for construction of the stereotactic space is the rigid application of a head frame to the patient’s skull. Stereotactic imaging is then performed with a localizer fitted over the patient’s head and attached to the frame. Fiducial markers in the localizer box define x, y, and z coordinates for any location in the defined space above the frame and appear on each image obtained. Imaging may be done with either computed tomography (CT) or magnetic resonance imaging (MRI) technology, or both with “fusion” of the two imaging modalities (Fig. 25-1).
Stereotactic Radiosurgery Delivery Systems
At present, radiosurgery is performed by using one of three methods of delivery of high-energy radiation. The radiosurgery technology most commonly available around the world uses high-energy x-rays generated from a clinical linear accelerator, or “linac.” Gamma ray radiosurgery with the gamma knife, although not as widely available as linac radiosurgery, is performed at an increasing number of centers and has been used more often than the other radiosurgery technologies. Radiosurgery with charged particles (e.g., protons or heavy ions) produced by a cyclotron or synchrotron is the technology least frequently used, but may be most suitable for very large target volumes.9 Any radiosurgery treatment-planning system should provide rapid, three-dimensional visualization of the target and isodose distribution superimposed on CT scans, MRIs, or angiograms.
Gamma Knife
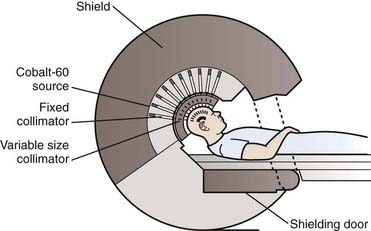
More than 250 gamma knife units are now located around the world, including more than 100 in the United States. As of the year 2006, more than 400,000 patients had been treated with this technology. The gamma knife unit consists of a central core containing either 192 or 201 cobalt 60 (60Co) sources surrounded by cast-iron or tungsten shields and a steel entrance door (Fig. 25-2). The sources are situated in individual beam channels in the central core directed convergently toward a single target point (isocenter) at the hollow center of the radiation unit (Fig. 25-3). Collimation of the beams is provided by collimators projecting to 4, 8, 14, or 18 mm diameter (Model 4C) or 4, 8, 16 mm (Perfexion) at isocenter (Fig. 25-4).
(From Lutz W: Radiation physics for radiosurgery. In Alexander E, Loeffler JS, Lunsford LD (eds): Stereotactic Radiosurgery. New York, McGraw-Hill, 1993.185)

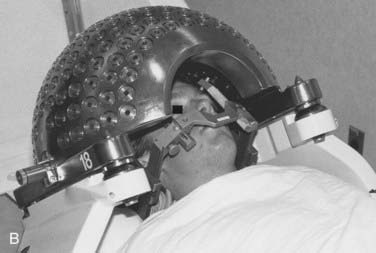
FIGURE 25-4 • A, Close-up of gamma knife helmet. The “8” designates this helmet as having collimators projecting to 8 mm at isocenter.
B, A patient positioned in a gamma knife helmet prior to entry into the gamma knife core.
After stereotactic frame placement and imaging, treatment planning consists of selection of a number of individual “shots” or radiation doses to small volumes within the target, each with a unique isocenter coordinate. Treatment plans are evaluated and optimized using dose-volume histograms (DVH). Once treatment planning is completed, the patient lies on the treatment couch, the position of which is adjusted so that the patient’s head, fixed within the stereotactic frame, is moved into the treatment position in the helmet (see Fig. 25-4). The frame is oriented within the helmet in the x, y, and z directions so that the radiation beams align with the target site according to the treatment plan. Several different isocenters may be treated in sequence during the treatment session to make the isodose surface conform closely to any irregular three-dimensional target contour (Fig. 25-5). Historically, it has required 10 to 15 minutes to place the patient in position and treat each isocenter. Modifications of the gamma knife have shortened these times considerably. Because 60Co has a half-life of 5 years, treatment times must progressively be increased.
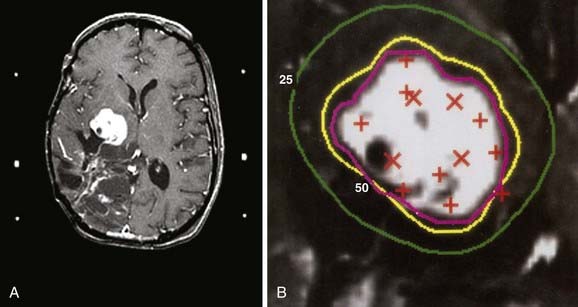
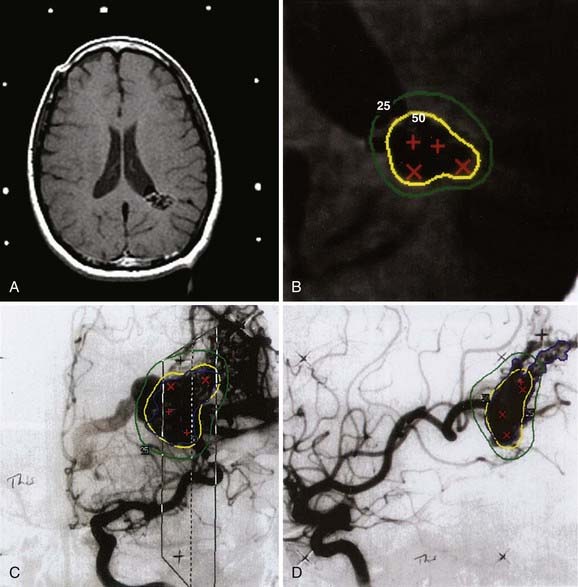
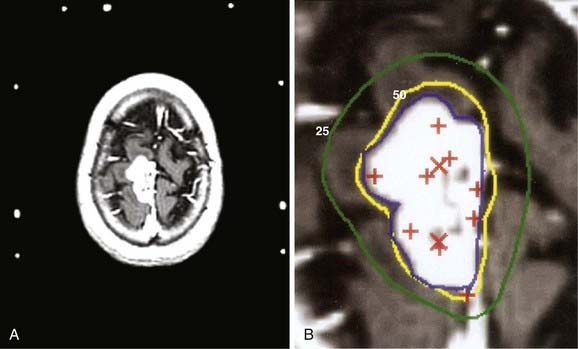
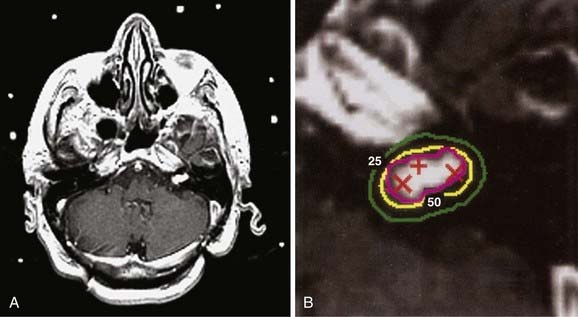
Figs. 25-6 to 25-8 demonstrate examples of gamma knife plans for arteriovenous malformation (AVM), metastatic disease, and a benign acoustic schwannoma, respectively. In each, the dose was prescribed at the 50% isodose line (yellow), which was required to conform tightly to the target configuration (red or blue). The 25% isodose line (green) is also shown. The crosses represent projections of isocenter locations onto the MRI plane.
Linear Accelerator Radiosurgery
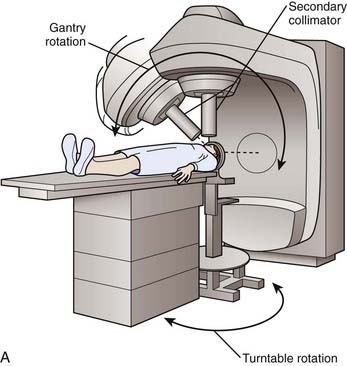
Like the gamma knife, linacs have a defined isocenter. The linac isocenter is the point of the intersection of the axis of rotation of the gantry and the treatment couch and the central axis of the photon beam (Fig. 25-9). Targets may be treated with single or multiple isocenter plans. Initial linac systems used special circular collimators of diameters from 5 to 60 mm to reduce the penumbra of the beam and the radiation dose to normal tissues. However, the use of circular collimators greatly limits the potential for isodose shaping, especially when using single-isocenter plans. Excellent conformity is now possible using micro-multileaf collimation (Fig. 25-10) and either multiple fixed fields or dynamic-arc radiosurgery. In the latter, the field shape is constantly changing to conform to the beam’s-eye view outline of the target volume (see Fig. 25-10). This treatment strategy has been shown to produce isodose distributions with conformity indices similar to those reported for multiple isocenter gamma knife plans.10
(A, From Lutz W: Radiation physics for radiosurgery. In Alexander E, Loeffler JS, Lunsford LD (eds): Stereotactic Radiosurgery. New York, McGraw-Hill, 1993.185)
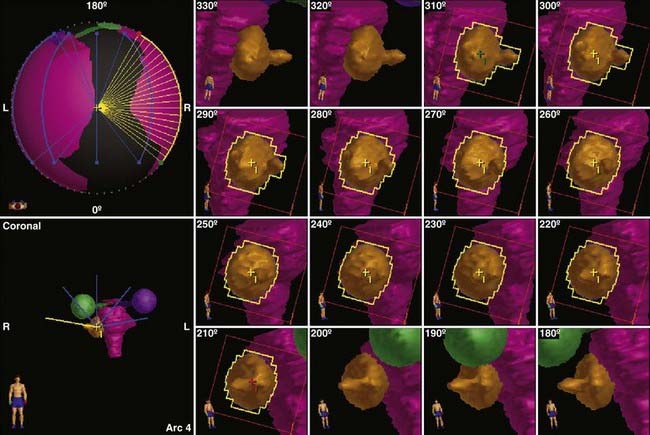
FIGURE 25-10 • Dynamic conformal arc (DCA) technique for linac radiosurgery. Right acoustic schwannoma was treated with 5 DCA. The micro-multileaf collimation (mMLC) conforms to the tumor periphery without a margin. The shape of the mMLC changes with each degree of arc. The resulting plan is shown in Fig. 25-11.
Figs. 25-11 to 25-13 demonstrate linac-based radiosurgery plans for acoustic neuroma, recurrent pituitary adenoma, and AVM. All plans employed a single isocenter.
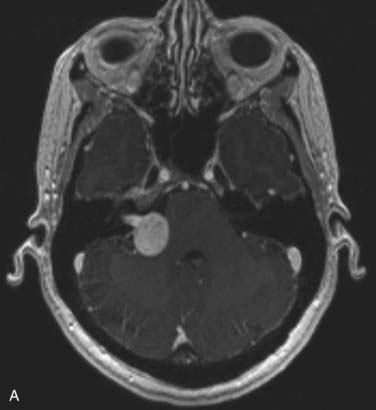
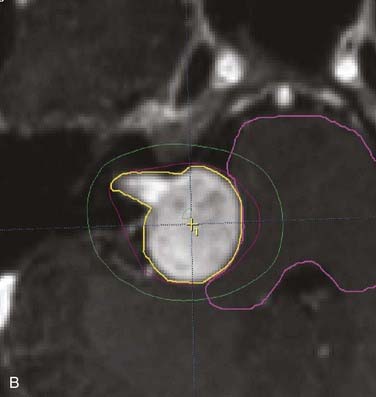
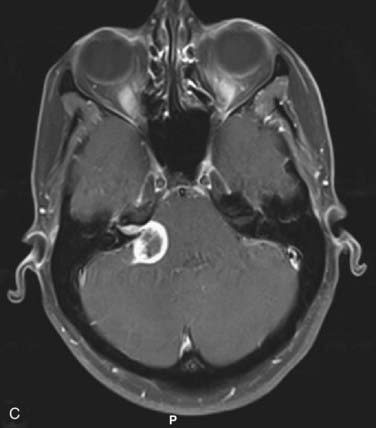
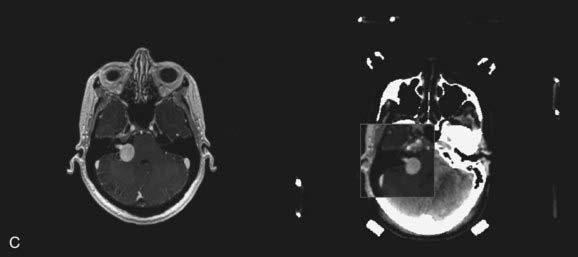
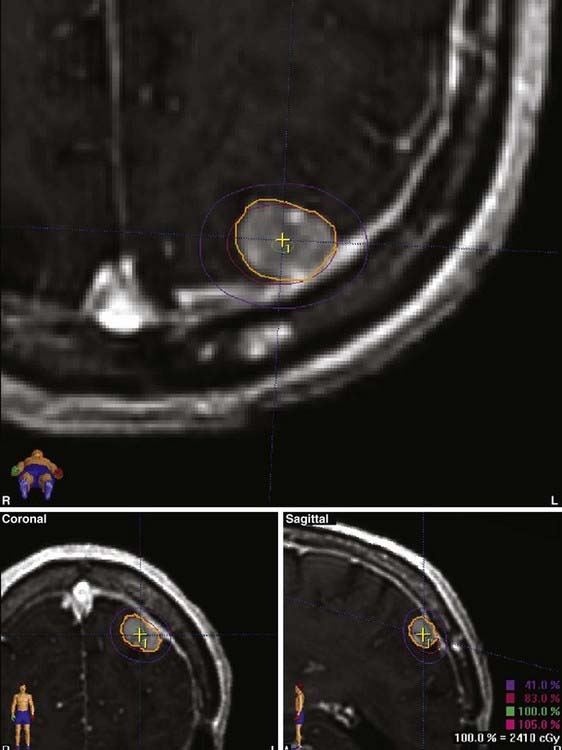
FIGURE 25-11 • A, Acoustic neuroma treated as shown in Fig. 25-10. Pretreatment magnetic resonance imaging (MRI) scan (left). The tumor volume was 4.1 cm3. B, A dose of 13 Gy was prescribed to the tumor margin corresponding to the 85% isodose line (red). The prescription isodose volume also contained 0.96 cm3 of normal tissue resulting in a conformity index of 1.3. Also shown is the isodose line corresponding to 50% of the prescription dose (green). C, A follow-up MRI scan 18 months after stereotactic radiosurgery shows smaller tumor volume and loss of central enhancement.
Further improvement in conformity of linac radiosurgery plans may be obtained using intensity-modulated delivery methods. Coupled with inverse treatment planning systems, avoidance of normal structures adjacent to the target volume is also possible.11 Clearly the future of linac-based stereotactic radiotherapy and radiosurgery lies in the ability to produce conformal treatment plans entirely covering target while using intensity modulation to avoid adjacent critical structures.12 Such treatment may often be delivered through a single isocenter and with prescription to the 80% isodose line or higher, thereby reducing dose inhomogeneity within the target.10
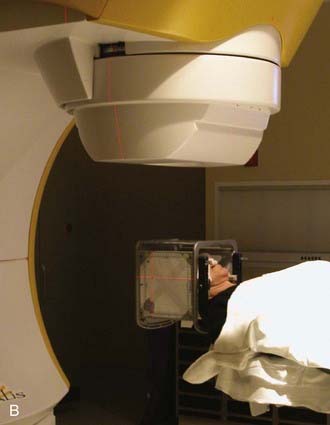
To align the patient’s head with respect to the linac beam, some groups have the patient lie on the treatment couch with the head supported and positioned on a separate, floor-mounted, stereotactic fixture, whereas others use only the treatment couch together with the standard alignment lasers associated with linac technology. Most groups recommend that target positioning be verified radiographically before each individual treatment. With most systems, patients are treated while they lie supine. In most cases, beam-entrance patterns trace a series of non-coplanar arcs on the scalp, one arc for each of several positions to which the table is turned.13
Particle-Beam Radiosurgery
Charged particle-beam radiosurgery has been used predominantly in North America, Europe, and the former Soviet Union during the previous 40 years, and during that time more than 6000 patients have been treated, some receiving therapy in as many as four fractions rather than one.14 In contrast to x-ray or gamma knife techniques, which require a large number of convergent radiation beams to produce a highly focal dose distribution, charged-particle beams produced by cyclotrons or synchrotrons produce a highly focal dose distribution with only a few beams because of the Bragg ionization peak produced by each individual beam. The width of the Bragg peak and its depth in relation to the scalp are adjusted to match the target’s dimensions and depth in brain. Patients usually are treated with two to six stationary, intersecting beams, each with its own irregularly shaped collimator that conforms to the contour of the target (Fig. 25-14). To ensure an acceptable three-dimensional dose distribution, compensators may be interposed in the beams to compensate for skull curvature and tissue inhomogeneities. As in linac or gamma knife radiosurgery, the three-dimensional dose distribution produced by a few intersecting particle beams falls off rapidly with distance from the target periphery, but it is usually more homogeneous within the target. Although proton radiosurgery is used to treat the common intracranial targets of photons techniques, a disproportionate number of patients with large irregular shaped lesions are referred to proton centers to achieve a greater conformality of dose. For example, at the Massachusetts General Hospital more than 50% of the AVMs treated are larger than 10 cc. This experience has provided unique data concerning dose-volume analyses for larger targets that is otherwise not available from photon radiosurgery experiences.15
Radiobiologic Effect
Usually, radiosurgery delivers little or no clinically important radiation dose beyond the target volume, in part because of the steep dose gradient at the target’s margins and in part because the minimum target isodose contour (usually 50% to 80%) conforms closely to the three-dimensional configuration of the target. Therefore, radiosurgery doses are high and inhomogeneous. The biologic effectiveness of radiation depends on the total dose of radiation, the dose per fraction, and the type of tissue irradiated. For late-responding tissues like normal central nervous system or vasculature tissue, there is a strong dependence on dose per fraction. With radiosurgery, total dose and dose per fraction are high, and variations in both according to isodose distributions produce sharply changing biologic effects. Within the target periphery, dose inhomogeneity (sharply rising dose from target margin to isocenter) has a disproportionate radiobiologic effect and may substantially elevate the potential for a cure or complication. Beyond the target’s perimeter, the radiobiologic effect declines rapidly as a result of decreasing dose and dose per fraction, a characteristic that may spare normal tissue, but that may permit the survival of potential infiltrative disease just outside the radiologically apparent target.16
Intracranial Target Lesions
Intracranial targets for radiosurgery fall into one of four categories based on two criteria: (1) whether the target tissue’s radiobiologic response to radiosurgery is early or late; and (2) whether the target volume contains any normal tissue.16
Category D
For small targets in categories A and B, it is to be anticipated that the ratio of patients cured to those with complications (therapeutic ratio) will bear little relation to the fractionation scheme used, whether single or multiple. For AVMs (category A), clinical data on fractionated treatments to a dose sufficient to test this prediction are not available. The clinical data available so far on radiosurgery for meningioma (category B) are not dissimilar to the best reported results of fractionated radiation therapy planned with CT or MRI.17–19 For metastases (category D), it might be thought that fractionated treatments would be preferable to single-fraction treatments because hypoxic tumor cells might reoxygenate and become more radiosensitive between fractions. The therapeutic ratio appears to be better with radiosurgery for metastatic lesions than with fractionated treatment, although a valid comparison is not possible because no studies have used sufficiently high fractionated doses. Why fractionation is not more important for these targets is not entirely understood, although the explanation may relate to the treatment volume. Usually, for small targets, a single fraction does not produce enough damage to normal tissue to have clinical consequences. In any case, most patients with lesions in category C and many in category D, except those with recurrent tumors, undergo fractionated irradiation as part of their therapy.
Single-fraction SRS results in tumor control rates for malignant tumors higher than predicted by modeling based on in vitro and in vivo killing of tumor cells by radiation.20 It has been proposed that, in the setting of single, high doses of radiation, the vascular endothelial cells become an additional important therapeutic target. Radiation-induced apoptosis of the endothelial cells may only become significant in single doses larger than 8 to 11 Gy.21 Similar effects likely come into play in the treatment of benign neoplasms with SRS.
Target Volume Definition
The hallmark of SRS is conformity of the prescription dose volume to the target volume. In conventional radiotherapy the gross target volume (GTV) defines radiographically visible tumor; there is often a “margin” added to this for microscopic extension, lymph nodes at risk, and so on, to form the clinical target volume (CTV); a further expansion is made to account for small, day-to-day errors in patient set-up to form the planning target volume. In SRS, the GTV is defined on stereotactic CT or MRI scan. In principle there is no need for expansion to cover subclinical disease or for set-up error. Set-up errors for all radiosurgery delivery systems are in the sub-millimeter range. Therefore, target volume definition is a crucial first step in the SRS planning process. Definition of AVM nidus or tumor volume requires the use of the appropriate imaging modality or modalities. Definition of normal structures at risk requires equal care. DVH analysis is highly recommended for evaluation of SRS plans in terms of target coverage and dose to normal structures. Various indices have been described to evaluate the degree conformity of the prescription isodose to the target volume.10,22
Radiation Dose
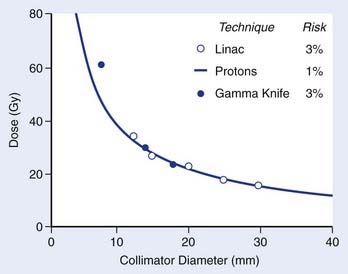
In all radiosurgery techniques, the volume of normal brain tissue beyond the target that receives a moderate to high dose of radiation increases approximately as the cube of the average diameter of the target volume. As complications of irradiation affecting normal tissue may correlate with the volume of normal tissue receiving a dose of a specified magnitude, at least to some extent, isoeffect dose levels may be expected to follow a power law, by which a log-log plot of isoeffective dose versus target diameter approximates a straight line with negative slope. The empirical power law dose-diameter isoeffect curve developed by Kjellberg and colleagues23 for proton radiosurgery is thought to correspond to a 1% risk for radiation necrosis of human brain tissue. The theoretical integrated logistic model dose-diameter curve developed by Flickinger24—obtained from a mathematical model that predicts the relationship of dose to volume for a 3% risk for radiation necrosis—closely resembles Kjellberg’s 1% curve for both gamma knife and linac radiosurgery. In each case, a log-log plot of isoeffective dose versus diameter approximates a straight line with negative slope. Although insufficient clinical data are available to confirm the level or slope of these curves, no doubt several variables, in addition to dose and volume, influence the probability of radiation damage. Still, Flickinger’s model can be helpful in comparing treatment plans to determine, for each particular patient, which plan has the least potential to cause complications (Fig. 25-15).
With the possible exception of AVMs and trigeminal neuralgia (see following), there are no clear dose-response data for targets treated with SRS. Most centers base doses on tumor volume and any restriction based on adjacent normal structures such as optic chiasm. Radiation Therapy Oncology Group (RTOG) 90-05 determined the maximum tolerated dose (MTD) of SRS in previously irradiated patients being treated for recurrence.25 Dose escalation was performed with stratification of patients into three categories based on maximum tumor diameter: less than 20 mm, 21 to 30 mm, and 31 to 40 mm. As expected, patients with smaller tumors tolerated higher doses. For the group with tumors smaller than 20 mm, 24 Gy was established as safe. Further dose escalation in this group was not possible because of lack of patient accrual at the 27-Gy level (presumably because of the reluctance of treating physicians to treat patients at this dose.) MTDs of 18 Gy and 15 Gy were established for patients with tumors between 21 and 30 mm and between 31 and 40 mm in diameter, respectively.
Although these results are extremely valuable and may serve as guidelines, several factors should be kept in mind when choosing SRS dose.26 First, patients in RTOG 90-05 were previously irradiated patients. Patients receiving SRS as a boost, immediately following whole-brain radiation therapy (WBRT), for example, for metastatic disease, may neither require nor tolerate the doses established as MTD in the protocol. Some clinicians decrease doses by approximately 10% when treating as a boost compared with treatment with SRS alone. Other special circumstances may also apply. For example, it is clear that acoustic neuroma is well treated with doses well below the RTOG MTDs, and that these doses would result in unnecessary toxicity (see the following text).
As complications of radiosurgical treatment have been documented more thoroughly, there has been a general reduction in dose levels prescribed. Central to the practice of radiosurgery are four general principles: (1) Slowly proliferating tissues may not manifest a response for months or years after treatment; (2) a slow response does not necessarily indicate that the lesion is radiation-resistant; (3) the latent interval can be decreased by increasing the dose delivered; and (4) response often reflects proliferative activity rather than intrinsic radiation sensitivity.27 Radiosurgical targets that are slowly proliferative include AVMs and benign tumors (meningioma, pituitary adenoma, and acoustic neuroma). Those lesions and other slowly growing tumors usually do not require a radiation dose that unduly risks damage to normal tissue to produce a rapid response or tumor necrosis.
Results of Radiosurgical Treatment
Arteriovenous Malformations
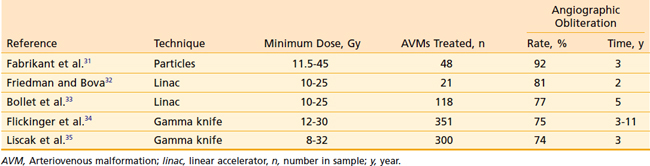
The natural history of AVMs represents a potentially life-threatening intracranial process, as is evident from the retrospective study of 160 patients with untreated AVMs (mean follow-up, 23.7 years) in which Ondra and associates28 found an annual rate of spontaneous hemorrhage of 3.9% and annual morbidity and mortality rates of 2.4% and 1.0% respectively. It appears that AVMs are cured, with little further risk of hemorrhage, when total microsurgical resection or total radiosurgical obliteration can be angiographically demonstrated.29 Because there seems to be a persistent risk of hemorrhage during the latent interval following radiosurgery, complete microsurgical excision of the AVM is the preferred therapy whenever it can be accomplished safely.30 When this is not possible, radiosurgery can obliterate both superficial AVMs and deep-seated, relatively inaccessible AVMs, with low morbidity and mortality rates and minimal hospitalization (Table 25-1). Radiosurgery induces a progressive thickening of the vascular wall and luminal thrombosis, which take months to years to complete. Reported obliteration rates are 74% to 92% at 2 to 3 years after radiosurgery (see Table 25-1).31–35 In most series, the obliteration rates at 2 years after radiosurgical treatment of small AVMs are higher (80% to 100%) than those for larger AVMs (40% to 70%).36 This has been shown to be due to the higher minimum doses delivered to smaller AVMs.37,38 Minimum doses of 13 and 25 Gy have been associated with in-field obliteration rates of 50% and 98%, respectively.38,39 The “gold standard” for success following SRS for AVM is angiographic evidence of complete obliteration. Patients may initially be followed with MRI until the nidus is no longer evident, at which time complete obliteration may be confirmed on angiogram.40 Repeat SRS for residual nidus demonstrated 2 to 3 years after SRS may result in subsequent obliteration in up to 85% of cases with low rates of morbidity.35,41,42 Likewise, an approach of repeat SRS for large AVMs may lead to improvement in obliteration rates, compared with a single session of low dose SRS.43
The rate of permanent residual neurologic injury after radiosurgery for AVM is 3% to 4%.35,42,44–46 In about one third of patients, follow-up MRIs show transient increased T2 signal changes surrounding the target that develop about 6 to 18 months after radiosurgery and resolve a year or so thereafter. These changes depend on the location and size of the target.47 Approximately one third of patients who develop these T2 signal changes also have transient neurologic symptoms.
Series treating patients younger than 18 years of age with radiosurgery for AVM have shown results comparable with those found in adults when similar doses are used. Smyth and colleagues48 reported on 40 children treated with SRS for AVM. Results were significantly correlated to marginal dose. Obliteration rates were 7% and 63% for marginal dose of less than 18 Gy and more than 18 Gy, respectively. Similarly, others have reported obliteration rates of 77% to 80% for small AVMs (<3 cc) and 61% to 65% for AVMs 3 to 10 cc in volume.49,50
Other related vascular lesions such as dural arteriovenous (AV) fistulae and cavernous malformations have also been treated with SRS. Dural AV fistulas treated in a fashion similar to AVMs (dose 10 to 28 Gy based on volume) demonstrated a 68% to 77% obliteration rate at 2-year follow-up. An additional 24% of shunts had significantly reduced flow, indicating a potential obliteration rate of up to 90% with further follow-up.51,52 Cavernous malformations have also been treated with SRS in selected “high risk” patients. Hasegawa and colleagues53 reported on 82 such patients who had experienced at least one major hemorrhage. During an average observation period of 4.3 years, the annual risk of hemorrhage was 33.9%. Following SRS (median dose 16.2 Gy), the risk of hemorrhage was reduced to 12.3% in the 2-year period after SRS and to 0.76% for years 3 through 12. Of these patients, 13% had new neurologic symptoms unrelated to hemorrhage following SRS; half of these were mild and transient. The authors recommend SRS for “high-risk” patients who have suffered at least one major hemorrhage.
Benign Tumors
Benign tumors commonly treated by radiosurgery are acoustic neuromas, meningiomas, and nonsecreting pituitary adenomas or those secreting somatotropin, adrenocorticotropin, or prolactin. Clinical observations on the radiosurgery of these lesions have provided fundamental information about the tolerance to single-fraction treatment on the part of the pituitary gland, the cranial nerves of the cavernous sinus, and the mesial temporal lobes.54–58
Acoustic Neuroma
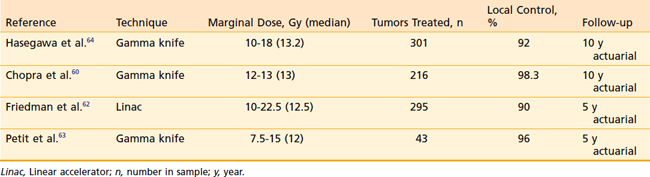
The role of radiosurgery for acoustic neuroma as an alternative to microsurgery is a topic of lively debate. The radiosurgical progression-free survival (PFS) rate with acoustic neuromas has been reported to be more than 90%.59 The early use of high doses in the range of 20 Gy at the tumor margin resulted in high rates of transient cranial nerve V and VII neuropathies. There was also hearing loss in approximately half of patients with useful hearing at the time of SRS. Because of these toxicities and the high rate of tumor control, doses have decreased during the past 10 years (Table 25-2). Chopra and colleagues recently reported a 98.3% 10-year control rate in more than 216 patients treated with the gamma knife to marginal doses of 12 to 13 Gy.60 No patient in this group developed a new facial neuropathy. Eight patients (3.7%) developed some degree of trigeminal neuropathy At 3 years there was a 78% probability of maintaining initial Gardner-Robertson hearing level in 110 patients with testable hearing. Similar results have been obtained by other institutions,61–64 and marginal doses in the range of 12 to 13 Gy have become the standard.
Follow-up imaging following SRS for acoustic schwannoma may show early enlargement of the tumor. Pollock reported a 14% rate of enlargement at a median of 9 months following SRS.65 The majority of these tumors (86%) did not subsequently demonstrate serial progression.
Fractionated stereotactic radiotherapy or staged radiosurgery has been used to treat acoustic neuromas with very similar results as those reported for SRS. Tumor control rates of more than 90% for treatment regimens of 50 to 54 Gy in standard fractions or 20 to 35 Gy in fractions of 4 to 5 Gy have been reported with virtually no incidence of cranial neuropathy and excellent preservation of hearing rates similar to low-dose SRS.66–69
Meningioma
SRS is increasingly becoming the sole treatment or a component of the treatment of intracranial meningioma. During the previous decade there have been a number of reported series with substantial follow-up demonstrating excellent local control of benign meningioma following SRS (Table 25-3).
Stafford and colleagues reported on 184 World Health Organization (WHO) grade-1 meningiomas in 168 patients treated with SRS.70 Local control at 5 years was 93% following marginal doses of 12 to 36 (median 16 Gy). These authors found no correlation between marginal dose and tumor control. Kreil and colleagues reported on 200 patients treated with a median dose of 12 Gy (7-25 Gy) SRS for meningiomas ranging in volume from 0.38 cc to 89.8 cc.71 Local control probability was 97.2% at 10 years with a complication rate of 2.5%, including temporary symptoms relating to peritumoral edema. Kollova and colleagues from the Czech Republic recently published their series of 325 patients with a median meningioma volume of 4.4 cc (0.11-44.9 cc).72 The median marginal tumor dose was 12.6 Gy (6.5-24 Gy). Five-year actuarial tumor control was 97.9%, with 69.7% of tumors showing some regression and 27.8% remaining stable in size. A strict dose limit was set for the optic pathways at 8 Gy for previously unirradiated patients and 3 Gy for previously irradiated patients. Temporary treatment-related toxicity was reported in 10.3% of patients and permanent effects were suffered by 5.7%. However, no patient was reported to have an optic nerve-related complication. Tumor control was significantly compromised when the marginal dose was less than 12 Gy. Patients treated with marginal doses in excess of 16 Gy had a significantly increased risk of peritumoral edema, temporary or permanent sequelae of radiosurgery. The authors conclude a dose range of 12 to 16 Gy for meningiomas is optimal. The University of Florida group treated 181 patients with WHO grade I meningioma with linac-based radiosurgery. A median marginal dose of 13.14 Gy (10-20 Gy) was delivered to a median treatment volume of 7 cc (0.3-48.6 cc). The local control rate was 96% at 5 years.73
Often meningiomas are treated without biopsy, based on characteristic appearance on MRI scan. Flickinger and colleagues74
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree