Fig. 16.1
Pulmonary complications after HSCT (borrowed from Gosselin and Adams 2002). HSCT hematopoietic stem cell transplantation
1.
First, important definitions:
a.
Consolidation: increased attenuation of lung parenchyma with complete obscuration of normal lung architecture/blood vessels.
b.
Ground glass: increased attenuation of lung parenchyma through which blood vessels/normal lung architecture appear indistinct but still visible.
c.
Air bronchograms: visualization of air-filled airways surrounded by consolidated lung parenchyma.
d.
Halo sign: a zone of ground-glass attenuation surrounding a pulmonary consolidation/nodule/mass.
e.
Nodule: increased attenuation on radiograph that is ≤ 3 cm in greatest dimension
f.
Mass: increased attenuation on radiograph that is > 3 cm in greatest dimension.
g.
Reticulation: fine curvilinear opacities.
h.
Infarct: consolidation that does not enhance and does not have air bronchograms, most of the time in peripheral distribution.
2.
Neutropenic phase (0–30 days)
During this period, patients essentially have no effective immune system and therefore are susceptible to a wide range of infections. Supportive care and empiric antibiotic therapy are important in successful passage through the early post-transplant period. The most common noninfectious complications during the neutropenic phase are pulmonary edema, drug toxicity, and diffuse alveolar hemorrhage (DAH) (Gosselin and Adams 2002):
a.
Pulmonary edema
Pulmonary edema, both cardiogenic and noncardiogenic, is very common in the immediate post-HSCT period. Patients receive large volumes of fluid in the form of medications, blood products, total parenteral nutrition, etc. This large-volume fluid infusion is further confounded by cardiac and renal impairment (consequences from chemotherapy administration) and concomitant hypoalbuminemia:
i.
Cardiogenic pulmonary edema:
Clinical symptoms
Dyspnea
Orthopnea
Lower extremity edema
Weight gain
ii.
Radiographic findings (Fig. 16.2):
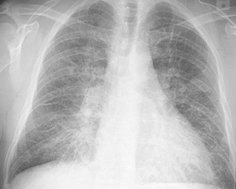

Fig. 16.2
Hydrostatic heart failure: The consolidation (water) is most severe in the lung bases symmetrically. Septal lines are seen throughout the lungs
Enlarged cardiac silhouette and pulmonary vessels.
Pleural effusions.
Septal and fissural thickening.
Ground-glass and consolidative opacities.
Of note, vascular indistinctness is best seen in the lung bases medially. The lungs are like two towels drying on a clothesline, the excess water collects mostly at the bases.
b.
Noncardiogenic pulmonary edema:
i.
Also called capillary leak/increased capillary permeability edema
ii.
Factors other than elevated intravascular pressure result in fluid and protein accumulation in the lungs, commonly including:
Total body irradiation
Multiple transfusions
Drug toxicity
Sepsis
iii.
May also be associated with engraftment syndrome (see Chap. 14) which manifests as:
Fever
Erythrodermatous skin rash
Noncardiogenic pulmonary edema
iv.
Radiographic findings (Fig. 16.3):
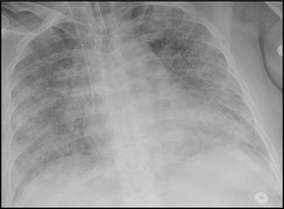

Fig. 16.3
Noncardiogenic edema: Diffuse ground glass reflecting the systemic cause of the vascular injury throughout the lungs, sepsis in this case
Diffuse symmetric consolidative and ground-glass opacities.
Typically, there is no clinical or radiographic evidence to suggest a cardiac etiology (cardiomegaly, septal lines).
The diffuse distribution is a very important clue, reflecting the systemic causes that induce the vascular leak throughout the lungs, as opposed to that of hydrostatic edema (which affects lower lobes predominantly).
The accumulation of fluid and protein in the lung tissue leads to decreased diffusing capacity, hypoxemia, and dyspnea and as a result, response to fluid restriction and diuresis is minimal.
c.
Drug toxicity:
i.
Drug toxicity can occur during both the neutropenic and early post-HSCT periods; see Sect. 16.3a for discussion.
d.
DAH:
i.
In the early history of HSCT, DAH was identified in as many as 20 % of patients. Currently, DAH is relatively uncommon and occurs most frequently in the 2–3 weeks post HSCT. It remains associated with very high mortality.
ii.
The exact pathophysiology of DAH is unclear but risk factors include:
Age > 40
Severe mucositis
Solid malignancy
Rapid neutrophil recovery
Allogeneic HSCT
Grades III–IV acute graft-versus-host disease (GVHD)
Conventional myeloablative transplant
iii.
Typical presentation:
Acute onset of dyspnea
Cough
Hypoxemia
Occasional hemoptysis
Fever
iv.
Radiographic findings:
Rapidly progressive consolidative and ground-glass opacities.
Septal thickening.
Sparing of the most peripheral aspects of lung parenchyma.
Absence of pleural effusions.
v.
Definite diagnosis requires bronchoalveolar lavage that demonstrates increasingly bloody return without identification of any infectious organism. Hemosiderin-laden macrophages are seen in lavage fluid.
e.
Bacterial/fungal infections:
i.
Pulmonary manifestations of bacterial and fungal infections are not commonly seen during the neutropenia period despite high prevalence of bacteremia.
ii.
The common empiric use of broad-spectrum antimicrobial agents may prevent development of infectious pneumonia.
iii.
Incidental septic emboli, which on radiography manifest as peripheral poorly marginated nodules which rapidly cavitate, is an exception.
iv.
Radiologic manifestation of other pulmonary bacterial and fungal infections will be discussed in Sects. 16.3.b and 13.3.c, as they are more common during the early/late post-transplant period.
f.
Acute GVHD:
Historically, pulmonary complications were thought to be a common complication of acute GVHD. Typically, other organ systems (skin, liver, and gut) are involved prior to lung involvement. Currently, acute GVHD involving the lung is considered a very rare event.
3.
Early phase (1–3 months) and late phase (3 months–1 year)
During the early period, 1–3 months after HSCT, the most common pulmonary complications are drug toxicity (allogeneic and autologous), and invasive aspergillosis and cytomegalovirus (CMV) pneumonia (allogeneic).
The late post-HSCT period extends from 3 months to 1 year. During this time, immune function continues to recover, and there is a reduction in pulmonary complications in autologous and syngeneic HSCT recipients. In contrast, allogeneic HSCT recipients often develop chronic GVHD which increases their risk of other infectious and noninfectious pulmonary complications:
a.
Get Clinical Tree app for offline access

Drug toxicity:
i.
Occurs most often in the first 100 days post HSCT.
ii.
Multiple cytotoxic mediations, such as carmustine, busulfan, and methotrexate, as well as radiation injury to the lung parenchyma are common offending agents.
iii.




Clinically, patients present with dry cough, dyspnea, and low-grade fevers.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



