8 Radiologic Techniques for Early Detection and Diagnosis
Film Mammography
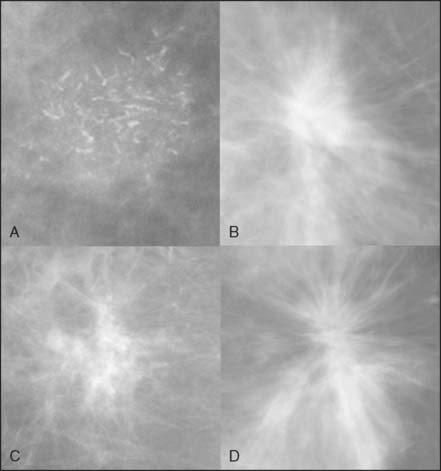
Despite no shortage of research on technologies meant to supplant it, mammography remains the mainstay of breast cancer screening. As such, any chapter on the early detection of breast cancer by imaging needs to include a discussion of this very important modality. A mammogram is an x-ray image of a compressed breast. For screening mammography, two mammograms are acquired in projections that are roughly 45 degrees apart. The signs of breast cancer on mammography include masses, architectural distortions, densities, and grouped calcifications (Fig. 8-1).
First proposed for use in widespread screening in the 1960s, mammography is the only screening test proven by randomized clinical trials to reduce breast cancer mortality. Eight large trials have been conducted to study the efficacy of screening mammography.1–3 The results of these trials show an average 30% reduction in breast cancer mortality rate. Despite the success in the overall cohort, there remains a longstanding debate on the usefulness of screening average-risk women between 40 and 50 years old.1,4,5 Although no trial had sufficient power to prove a significant difference in mortality for this age group, secondary analyses and meta-analyses have been put forward to support the use of screening mammography in this age group.6–7 Currently, annual screening starting at age 40 is the recommendation of many professional organizations, including the American Cancer Society.8
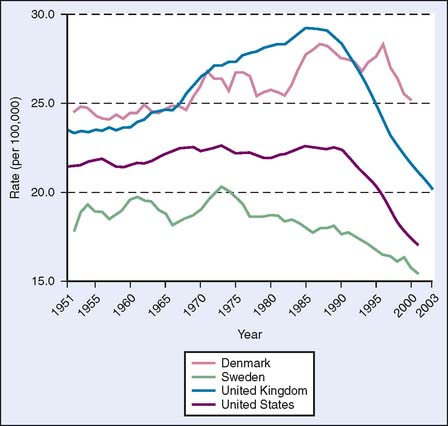
Data supporting screening mammography can also be gleaned from population statistics on breast cancer mortality rates. In countries with screening programs, including the United States, Sweden, and the United Kingdom, decreases in breast cancer mortality are seen starting about 10 years after the initiation of widespread mammographic screening, in the mid-1970s in Sweden and in the mid-1980s in the United States and the United Kingdom.9 In Denmark, however, which is just starting a screening program, breast cancer mortality rates continued to increase in that same period (Fig. 8-2), although there has been a decrease in Copenhagen, where a screening program was started in 1991.10
Digital Mammography
A much publicized development in mammography is the advent of digital mammography. This technology replaces the phosphorescent screen and film used in standard mammography with a digital detector. The advantages of the digital detector are greater contrast resolution and greater dynamic range, meaning that it is able to distinguish finer shades of gray in all parts of the image—light and dark. Digital mammography also enables digital image manipulation, as well as digital storage and retrieval. The disadvantage of digital mammography is a slightly lower spatial resolution. In addition, although digital mammography provides faster patient throughput per machine than film, it takes longer to interpret each study, primarily owing to the extra time spent manipulating the images.11
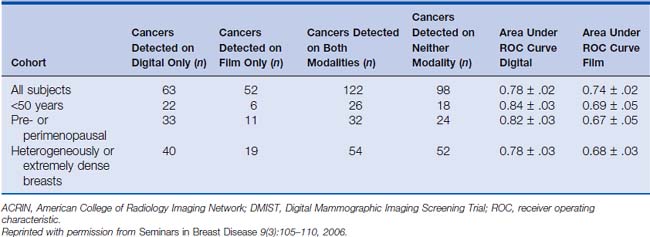
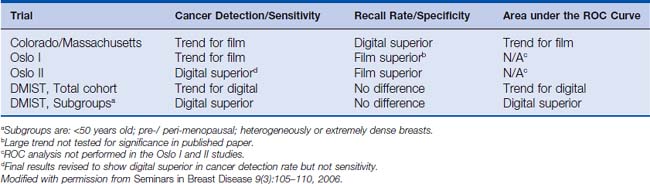
Because of both technical advantages and disadvantages of digital mammography compared with film mammography, clinical trials have been conducted to test the two modalities for cancer screening. Four prospective trials have been conducted to date. Three of these were paired trials, in which each subject received both a film and a digital mammogram, allowing the subject to be used as her own control. The first two of these trials, the Colorado/Massachusetts trial and the Oslo I trial, showed no significant difference between film and digital mammography; in fact, both trials had trends favoring film for cancer detection (although both showed lower call-back rates for digital).12,13 The most recent and largest paired trial, the Digital Mammographic Imaging Screening Trial (DMIST), conducted by the American College of Radiology Imaging Network (ACRIN), though not showing an overall difference, did show a significant advantage of digital mammography in certain subgroups of women, namely, premenopausal women with dense breasts under 50 years of age.14,15 This is intuitively satisfying, since it is within dense breasts that one would expect the superior contrast resolution of digital mammography to have the greatest benefit. Table 8-1 summarizes the results of the DMIST trial. The one randomized trial, the Oslo II trial, in which each subject was randomized to either film or digital mammography, originally showed no difference in cancer detection,16 but the final analysis was revised to show a statistically significant improvement in cancer detection rate, but not sensitivity, by digital mammography.17 This improved cancer detection rate was at the expense of an increased recall rate, however. Table 8-2 summarizes the results of the four clinical trials in digital mammography.
Table 8-2 Comparison of Overall Results for Four Major Digital Mammography Screening Trials12,13,15–17
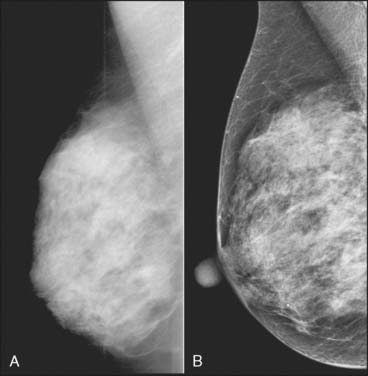
Despite no clear proven advantage in cancer detection for most women, digital mammography is replacing film mammography. Most new systems sold are digital. The primary reason centers switch to digital is operational. Since the rest of radiology has long since done away with film, and therefore film processors and film storage, it makes operational sense to switch mammography to digital and take advantage of the digital infrastructure now present in most departments. The digital nature of the data also enables sophisticated postprocessing that allows the tissue to be optimally displayed in both the high-density and low-density portions of the image. Combined with the improved dynamic range and contrast resolution, this processing seems to make it possible to see through dense tissue in a way that was not possible with film mammography. At the same time, the periphery of the breast can be lightened digitally so that it is easily seen without any brightness adjustment by the user. Figure 8-3 shows a comparison of a dense breast imaged on both film and digital mammography. There is no question that digital mammography can create beautiful depictions of masses and microcalcifications; the question is whether these visually appealing images actually translate into a clinical benefit.
Ultrasound
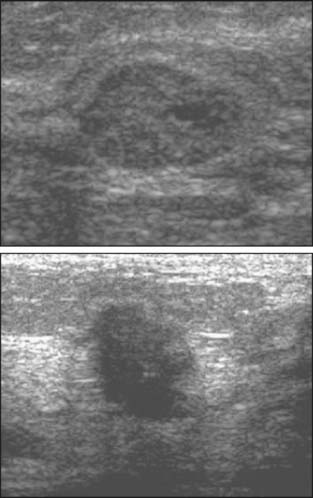
Over the past 15 years, ultrasound has progressively increased in importance as a modality for diagnosing breast cancer. Ultrasound is used as a diagnostic tool to work up both mammographic findings and palpable abnormalities. Ultrasound has been used for over two decades to distinguish cysts from solid masses, but it is now commonly also used to characterize solid masses (Fig. 8-4) and to confirm questionable mammographic abnormalities. Ultrasound now serves as the primary modality for directing image-guided needle biopsies of masses. Stereotactic mammographic imaging is still the primary guidance for biopsies of microcalcifications.
One of the greatest diagnostic strengths of ultrasonography is in evaluating palpable abnormalities. Most important is its high negative predictive value, which lies in the range of 99% to 100%.18–20 In other words, the likelihood that a palpable abnormality is cancer falls to below 1% if no abnormality is detected on ultrasound examination of the palpable area. This contrasts with the relatively poor predictive value of a negative mammogram in the presence of a palpable abnormality. Cases of delayed diagnosis of palpable cancers because of a negative diagnostic mammogram are well known. For this reason, imaging evaluation of a palpable abnormality should always include ultrasound.
Kolb and colleagues21 performed 13,547 breast screening ultrasound examinations in women with dense breasts and normal mammograms and physical examinations. Thirty-seven cancers were found in 34 women for a yield of 0.27%, a percentage similar to the yield of screening mammography. A large ultrasound screening trial is underway under the auspices of ACRIN.
Magnetic Resonance Imaging
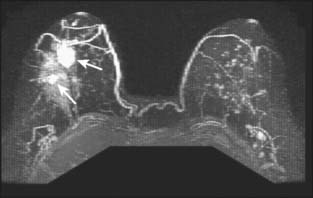
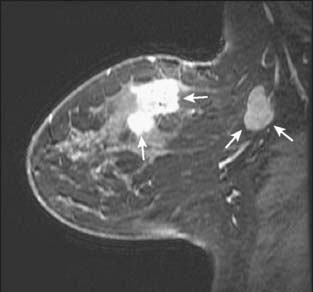
Contrast-enhanced magnetic resonance imaging (MRI) is a rapidly growing modality for evaluating the breast. The most common use at present is to evaluate the extent of disease in the ipsilateral and contralateral breast in a patient with a new diagnosis of cancer. MRI is superior to mammography and ultrasound in determining the size of the tumor, the presence of multifocal or multicentric disease (Fig. 8-5), and the presence of contralateral disease. In addition, MRI shows enlarged lymph nodes suggestive of metastatic spread (Fig. 8-6). This use of the technology is becoming more common throughout the world, but it is not supported by any studies showing improved outcomes. A knowledge of the extent of disease in the ipsilateral breast should improve the success of lumpectomy in obtaining negative surgical margins. It is not clear, however, whether additional foci of disease away from the index lesion are a common cause of ipsilateral recurrence after breast conservation, since most such recurrence occurs at the lumpectomy site. A recent large multicenter trial looking at the contralateral breast found a 3% yield in detecting contralateral cancers.22 This figure is actually lower than those reported in smaller single-institution series.23,24 The difference is presumably due to the characteristics of the women studied. The earlier papers had a cohort skewed toward high-risk women, including those with BRCA mutations. Such patients are known to be more likely to have bilateral breast cancer. Of the 30 contralateral cancers in the multicenter trial, 12 were ductal carcinoma in situ (DCIS), and all but 1 of the 18 invasive cancers were smaller than 2 cm, with an average size of 9 mm. It is unknown how many of these cancers would have presented clinically.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree