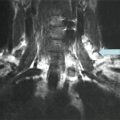
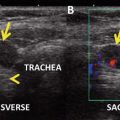
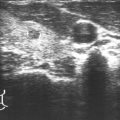
Fig. 29.1
Pretreatment I-123 scan in a patient with low-risk papillary thyroid cancer, idiopathic hyperprolactinemia, and mild galactorrhea demonstrating bilateral breast uptake. The decision was made not to treat this patient with radioiodine based on the scan
Risk of Secondary Malignancy Associated with RAI Therapy
The risk of second primary malignancy (SPM) after administration of RAI is well documented, but generally thought to be low. Although the risk of both leukemia and salivary gland malignancies is increased, there is no evidence of greater risk of breast cancer [11–13]. The risk of breast cancer among women with increased breast radioiodine uptake has not been examined.
Prevalence
A retrospective study performed from 2000 to 2006 to examine the utility of radioiodine scans prior to RAI therapy in patients with DTC showed that 6 % of non-lactating women had evidence of breast uptake on pretreatment scans [14]. Similarly, Hammami et al. demonstrated that approximately 6 % of all nonpregnant and non-lactating women (23 had not breastfed for an average of 11.4 months; four were nulliparous; three were postmenopausal) with iatrogenic hypothyroidism following thyroid hormone withdrawal also had breast uptake on radioiodine scans. The authors noted four different patterns of radioiodine breast uptake: “full,” “focal,” “crescentic,” and “irregular.” No association, however, could be made between scintigraphy patterns and etiology of breast uptake. The majority of women did not have expressible galactorrhea (52 %) and were normoprolactinemic (76 %; the remainder had prolactin levels <2.5 times the upper limit of normal, likely due to iatrogenic hypothyroidism) [15].
Pathophysiology
Iodide is an essential component of thyroid hormones tri-iodothyronine (T3) and thyroxine (T4). Intracellular iodide stores are maintained by NIS , located on the basolateral plasma membrane of thyroid follicular cells. NIS expression facilitates the use of radioiodine for both diagnostic and therapeutic purposes in the management of thyroid cancer. Active iodide transport also occurs in extrathyroidal tissues, including the lactating breasts, salivary glands, small intestine, and stomach [4]. In lactating breast tissue, expression of NIS allows iodide to be concentrated and secreted into breast milk for neonatal nutrition. NIS is generally present in breast tissue only during gestation and lactation. This is likely due in part to a threshold level of estrogen necessary for both direct effects on mammary gland NIS transcription, as well as effects via oxytocin and prolactin [16]. Expression of NIS has also been found to be high in fibroadenomas and, to a lesser extent, malignant breast tissue [9].
Role of Prolactin
Hyperprolactinemia, even in the postmenopausal woman with baseline atrophic breast tissue, may also result in increased breast uptake on scintigraphy that is reversible with normalization of prolactin levels [7, 17]. Both animal models and cultured human breast cancer cells respond to prolactin stimulation with increased expression of NIS and thus radioiodine uptake [7].
Medical Therapy
False positive breast uptake should first be ruled out by taking a focused history with consideration of lateral radiographic views and SPECT imaging. Subsequently, if the decision is made to proceed with radioiodine therapy, breastfeeding should be discontinued, and/or offending agents resulting in hyperprolactinemia should be stopped if medically feasible. Little data exist regarding therapeutic options. Hsiao et al. published a case report of a single postpartum woman treated with bromocriptine with subsequent minimal breast uptake on scintigraphy 8 weeks after cessation of breastfeeding [18]. Brzozowska et al. reported an observational case series of eight postpartum women who were followed by I-123 scintigraphy; five women received dopamine agonist therapy (cabergoline or bromocriptine) and three women received no therapy. The duration of ongoing breast uptake on scintigraphy was quite variable, but lasted up to 32 weeks in women who did not receive dopamine agonist therapy. In contrast, women who received dopamine agonist therapy had negative uptake studies sooner (3–10 weeks in four of the five women in the treatment group) [19]. Although data are limited in this clinical setting, cabergoline is generally regarded to be more efficacious in the normalization of prolactin levels as compared to bromocriptine [20]. Thus, patients may be initiated on cabergoline 0.25 mg twice/week (titrated up to 1 mg twice/week) or bromocriptine 7.5 mg twice/day based on existing data. Although dopamine agonists may shorten the time interval between cessation of breastfeeding and radioiodine therapy, it is unlikely that a delay in the administration of I-131 will change the final outcome of patient. Diagnostic scintigraphy with an I-123 scan should be performed prior to administration of therapeutic I-131 to confirm absence of breast uptake [14, 19].
Management of the Case
Our patient qualifies for RAI therapy on the basis of her bulky primary tumor focus and metastatic disease. As she had stopped breastfeeding only 2 weeks prior, she was initiated on cabergoline 0.5 mg twice/week. Six weeks later, she underwent a pretreatment scan that showed no evidence of breast uptake. The patient was treated with 75 mCi of radioiodine and had a posttreatment scan showing two foci of uptake in the thyroid bed. She has done well subsequently without clinical, sonographic, or biochemical evidence of recurrent disease including a negative total body scan and an undetectable serum thyroglobulin after recombinant TSH nine months later.
Clinical Pearls/Pitfalls
Breast uptake on I-123 scintigraphy is possible in the setting of recent cessation of breastfeeding or in non-lactating women with hyperprolactinemia, breast cancer/tumor, or mastitis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree