Radiofrequency ablation (RFA), usually performed under percutaneous ultrasound guidance, is considered the gold standard among minimally invasive therapies. On the strength of some recent randomized trials, its indications include operable patients with small hepatocellular carcinoma and inoperable patients with more advanced disease also in combination with other therapies. RFA has lower complication rates and costs less than surgery.
Advances in diagnostic imaging and fine-needle biopsy technique have permitted the development of percutaneous ablation therapies (PATs). These therapies are performed using a direct image-guided approach through the skin and organ parenchyma. PATs may be based on the use of means capable of destroying the tissue chemically, such as ethyl alcohol (PEI) or acetic acid (PAI), or physically, such as laser, radiofrequency (RF), or microwave (MW). These techniques permit the destruction of tumors without necessitating their removal and, in some cases, can be used in place of more invasive and expensive surgical procedures.
PEI, the first PAT to be proposed, was independently conceived at the University of Chiba in Japan and at the Vimercate Hospital (Milan) in Italy. The first study in an international journal appeared in 1986. From its rationale and the results obtained, the other techniques were subsequently designed. Whereas for some years only patients with up to 3 small (3 cm in size) or single (<5 cm in size) lesions were treated, and this still applies at many centers, with the introduction of single-session procedures under general anesthesia, even patients with lesions greater in number or larger in size are now being treated.
RF ablation (RFA) is currently considered the gold standard among PATs because of its recent results. In the clinical field, RFA is used for the treatment of a variety of neoplasms, principally metastatic hepatic deposits, hepatocellular carcinoma (HCC) in cirrhosis, renal cell carcinoma, lung tumors, and osteoid osteoma.
This article considers RFA of HCC in patients with cirrhosis. In this disease, this minimally invasive therapy has the potential to dramatically alter patient outcome, because other existing therapies either are associated with significant morbidity, or have limited efficacy. In these patients, liver dysfunction and associated coagulopathy often combine to make surgical resection an unacceptably risky procedure.
Principles and techniques
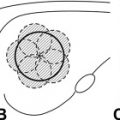
The treatment of thermoablation with RF exploits the conversion of the energy of an electromagnetic wave into heat. A generator is used that converts normal energy supplied by an electric alternating current of 90 Hz into the RF band of 500 KHz. The current is linked to an active electrode in the form of a needle, which is inserted into the tumor so that the body becomes part of the electric circuit, and is dispersed with a passive electrode in the form of a plate, which is applied to the skin of the patient. In this way, a resistive type of heating is produced, particularly around the exposed point of the needle electrode, caused by ionic agitation of the tissue electrolytes that follow the change in direction of the alternating current. Heat is generated by means of impedance (resistance) that the surrounding tissue opposes to the flow of current, so that heat is not generated at the tip of the electrode but within the tissue. The heat is produced by the difference between the heat generated around the extremity of the electrode and dispersed heat, the entity of which depends on the conductivity of the tissue and dissipation by convection caused by blood circulation (the so-called sink effect). In the presence of a physical and electrical homogeneity, the heat generated around the noninsulated extremity of the electrode is regulated by the distance from the tip, by the intensity of the current, and by the duration of the application. One potential limitation of conventional monopolar RF techniques is that the diameter of tissue coagulated is limited by heat dispersion to a maximum of approximately 1.6 cm. Increasing tip temperatures to greater than 100°C does not result in greater volumes of coagulation necrosis, because of tissue vaporization and charring. This situation leads to increased local tissue impedance and thereby limits RF deposition, heat diffusion, and coagulation necrosis. To overcome these limitations, several strategies have been proposed. These strategies include the use of bipolar electrodes, multiprobe arrays, saline injection during treatment, and cooled-tip electrodes.
RFA equipment
Several generators are commercially available, some of which have incorporated circuitry that enables measurements of generator output (wattage and milliamperage), tissue impedance, and electrode tip temperature. The most widely used instruments are made by 3 companies: RadioTherapeutics Corporation (Sunnyvale, CA, USA), acquired by Boston Scientific; RITA Medical Systems (Mountain View, CA, USA), acquired by AngioDynamics; and Radionics (Burlington, MA, USA), acquired by Covidien, a division of Tyco Healthcare Group. Each of the devices uses a different needle design, wattage, and algorithms. The first uses different LeVeen electrodes capable of obtaining ablations 2 to 5 cm in size with an expandable umbrella-shaped array design, 1.9 mm in diameter. The electrodes are available with array diameter ranging from 2.0 to 5.0 cm. The RF 2000 and the RF 3000 generators produce maximum powers of 100 and 200 W, respectively. The second produced different models with time. The Starburst ablation device has a unique patented umbrella-shaped array design with a live trocar tip, 9 electrodes, and produces real-time temperature feedback from 5 independent thermocouples within the array. The 1500X RF generator has a maximal 250-W power output. The generator is started at 25 W and slowly increased over a few minutes. Once the temperature is around 100°C, the hooks are fully deployed. The main difference between the 2 systems is that LeVeen model uses tissue impedance as feedback monitoring, whereas the RITA model relies on temperature. The third uses a single cold perfusion electrode with a diameter of 1.2 mm and the tip exposed for 2 to 3 cm. The power RF generator has a maximal 20-W power output and can display temperature as well as tissue impedance. By avoiding early increments of impedance linked to carbonization, these electrodes permit application of a greater power with respect to conventional electrodes. To obtain cooling, a physiologic solution brought to 2 to 5°C is circulated within 2 coaxial lumens situated in the electrode. The technique determines a reproducible area of necrosis of 2 to 4 cm in diameter. An electrode with a cluster of 3 cooled tips in a triangular pattern, permitting a higher current deposition, determines more than 4.5 cm of coagulation necrosis.
Two prospective trials compared the effectiveness of RFA performed by using different models for the treatment of HCC until 3.0 cm in size. Shibata and colleagues performed a comparison of Cool-tip single electrode and LeVeen electrode with 10 expandable hooks. There was no significant difference in local effectiveness, major complications, local tumor progression, and overall survival (OS). Lin and colleagues performed a comparison of the RF 2000 with LeVeen electrode, RF 3000 with LeVeen electrode, RITA with StarBurst electrode, and Cool-tip single electrode for the ablation of HCC. Similarly, there was no significant difference in the rate of volume necrosis obtained, in the rate of local tumor progression, in overall and disease-free survival at 2 years, between the groups.
Other instruments are made by Invatec (Concesio, Italy), recently acquired by Medtronic Inc, producing an electrode containing 1 or 3 spiral arrays that when deployed extend 2.0 cm beyond the tip, and Celon AG (Berlin, Germany), a member of the Olympus Medical System Group, producing a bipolar or multipolar electrode that avoids the use of the passive pad placed on the patient’s thigh.
RFA equipment
Several generators are commercially available, some of which have incorporated circuitry that enables measurements of generator output (wattage and milliamperage), tissue impedance, and electrode tip temperature. The most widely used instruments are made by 3 companies: RadioTherapeutics Corporation (Sunnyvale, CA, USA), acquired by Boston Scientific; RITA Medical Systems (Mountain View, CA, USA), acquired by AngioDynamics; and Radionics (Burlington, MA, USA), acquired by Covidien, a division of Tyco Healthcare Group. Each of the devices uses a different needle design, wattage, and algorithms. The first uses different LeVeen electrodes capable of obtaining ablations 2 to 5 cm in size with an expandable umbrella-shaped array design, 1.9 mm in diameter. The electrodes are available with array diameter ranging from 2.0 to 5.0 cm. The RF 2000 and the RF 3000 generators produce maximum powers of 100 and 200 W, respectively. The second produced different models with time. The Starburst ablation device has a unique patented umbrella-shaped array design with a live trocar tip, 9 electrodes, and produces real-time temperature feedback from 5 independent thermocouples within the array. The 1500X RF generator has a maximal 250-W power output. The generator is started at 25 W and slowly increased over a few minutes. Once the temperature is around 100°C, the hooks are fully deployed. The main difference between the 2 systems is that LeVeen model uses tissue impedance as feedback monitoring, whereas the RITA model relies on temperature. The third uses a single cold perfusion electrode with a diameter of 1.2 mm and the tip exposed for 2 to 3 cm. The power RF generator has a maximal 20-W power output and can display temperature as well as tissue impedance. By avoiding early increments of impedance linked to carbonization, these electrodes permit application of a greater power with respect to conventional electrodes. To obtain cooling, a physiologic solution brought to 2 to 5°C is circulated within 2 coaxial lumens situated in the electrode. The technique determines a reproducible area of necrosis of 2 to 4 cm in diameter. An electrode with a cluster of 3 cooled tips in a triangular pattern, permitting a higher current deposition, determines more than 4.5 cm of coagulation necrosis.
Two prospective trials compared the effectiveness of RFA performed by using different models for the treatment of HCC until 3.0 cm in size. Shibata and colleagues performed a comparison of Cool-tip single electrode and LeVeen electrode with 10 expandable hooks. There was no significant difference in local effectiveness, major complications, local tumor progression, and overall survival (OS). Lin and colleagues performed a comparison of the RF 2000 with LeVeen electrode, RF 3000 with LeVeen electrode, RITA with StarBurst electrode, and Cool-tip single electrode for the ablation of HCC. Similarly, there was no significant difference in the rate of volume necrosis obtained, in the rate of local tumor progression, in overall and disease-free survival at 2 years, between the groups.
Other instruments are made by Invatec (Concesio, Italy), recently acquired by Medtronic Inc, producing an electrode containing 1 or 3 spiral arrays that when deployed extend 2.0 cm beyond the tip, and Celon AG (Berlin, Germany), a member of the Olympus Medical System Group, producing a bipolar or multipolar electrode that avoids the use of the passive pad placed on the patient’s thigh.
Procedure
RFA is generally performed percutaneously under ultrasound (US) guidance, because this real-time control allows faster execution, precise centering of the electrode on the target, continuous monitoring of distribution of vapor bubbles, and determination of the appropriate amount of energy to give each time. A hyperechoic focus, which represents microbubbles of gas that form in the heated tissue, increases in size during the procedure, starting from the distal part of the uninsulated tip and then occupying the target. This region can be variable in size and irregular in shape and contour, and grossly reproduces the area of necrosis obtained. This hyperechogenicity obscures the tumor for 10 to 15 minutes after treatment, and sometimes makes it difficult to reposition the electrode for further insertions in brief time. Second-generation US contrast media injected after treatment are useful to decide on persistence of viable tissue. In some centers, principally in the United States, the procedure is performed under computed tomography (CT) guidance. CT guidance does not permit real-time control and precise centering is longer. However, contrast-enhanced CT shows regions of hypoattenuation devoid of characteristic parenchymal enhancement immediately after ablation, representing the areas of necrosis obtained.
In our center, the therapy plan foresees the completion of treatment in only 1 session, with an eventual retreatment after the first control of therapeutic efficacy. Because the procedure may be painful, it is performed under sedation/analgesia when 1 or 2 insertions are foreseen (in lesions <2 cm), or under general anesthesia with tracheal intubation when a greater number is planned. RFA is performed with real-time US guidance. A guide device incorporated into the US probe is used for electrode placement, permitting different angles of inclination, from 0 to 30°. After cleansing of the skin with iodized alcohol, which also serves as contact medium, the most appropriate approach for electrode insertion is selected. For lesions located in the right lobe, an intercostal approach with the patient in the left lateral decubitus position is generally preferred. For lesions located in the left lobe, a subcostal approach is used most often. The goal of therapy is to destroy the nodule and 0.5 cm of tissue surrounding it. The more the tumor increases in size, the more difficult it is to obtain a safety margin. Using the appropriate number of insertions it is not difficult to reach the complete necrosis of the nodule, even in cases larger than 5 cm, because of the so-called oven effect, which obtains large volumes of necrosis into neoplastic tissue, greater than into cirrhotic tissue. Usually we use 18-gauge internally cooled RF electrodes, single, with 2.0 or 3.0 cm of exposed metallic tip. The appearance and progression of hyperechogenicity is used to guide the duration of therapy. RF is applied until the tumor and the safety margin (when possible) surrounding it appears completely hyperechoic, and the hyperechoic focus does not increase in size for some minutes. Furthermore, in cases in which multiple insertions are required, each subsequent electrode placement is directed to an area of the tumor where hyperechogenicity is not evident. Each application of RF energy lasts 8 to 12 minutes, and in all cases the entire treatment is less than 1 hour. After therapy, patients are hospitalized for another day. Similar procedures are performed elsewhere with outpatients.
This article reports some important practical points suggested by Rhim and colleagues, from one of the leading centers in this field, for obtaining a successful RF ablation, a lesson learned from 3000 procedures. We have an equivalent experience in number of patients treated, and agree with this kind of patient management. We divide Rhim and colleagues’ suggestions into 3 categories: best planning, safe ablation, and complete ablation.
- 1.
“Planning includes the following: (i) to assess feasibility of procedure based on inclusion/exclusion criteria; (ii) decision of type of approach (ie, percutaneously, laparoscopically, open), electrodes, guiding modalities and number of ablations; (iii) to decide whether to apply overlapping ablations or a special technical tip (eg, artificial ascites) for safe and complete ablation. The feasibility assessment for RFA begins with a review of good quality CT or MR [magnetic resonance] imaging. These preoperative studies are used to determine the number and size of tumors and their relationship to surrounding structures including blood vessels or vital organs. An operator should perform planning US if a US-guided procedure is considered. An operator should assess size of the tumor, whether there is a safe electrode path to the tumor, whether there is any organ close to the expected RFA zones, whether there is any large vessel close to the tumor and whether there are factors favorable for percutaneous approach. Thus, an operator should consider an alternative approach, alternative guiding modality or alternative treatment modalities (eg, PEI, PAI, TACE [transcatheter arterial chemoembolization]) depending on the degree of feasibility or the cause of technical difficulty. HCC generally requires more than a single treatment modality. Hence, we should select the proper treatment modality based on the status of the initial and recurrent tumors. Tailor-made ablation strategies with multimodality treatments may be the best approach for successful ablation outcomes.”
- 2.
“Minimal invasiveness is a clear advantage of image-guided ablation over the surgical treatment. Safe ablation can be supported by careful patient selection, close patient monitoring during the ablation and appropriate management of complication after the ablation. As careful patient selection cannot guarantee safe ablation, early detection during or immediately after ablation is more important for managing the major complications appropriately. To minimize the mortality resulting from the major complication, an interventional radiologist should be aware of the broad spectrum of complications encountered after RFA of hepatic tumors (eg, bleeding, massive infarction, extensive abscess with sepsis and thermal injury of the colon). The frequency of major complications may be correlated with both the experience and aggressiveness of an operator. If an operator has more aggressive posture for achieving complete ablation with enough ablative margin, the rate of major complication will be increased even treating technically feasible tumors. Although mild bowel edema or bile duct dilatation adjacent to the RFA zone was often seen at CT performed during or immediately after follow up, a major complication requiring specific treatment was very rare. However we recommend alternative treatment, especially for tumors broadly abutting colonic loops or major central bile ducts. All technical tips to minimize the complication should be considered if there is any possibility of complication at planning base. There are several technical tips to minimize collateral injury to vital organs including: (i) a different approach ; (ii) using artificial fluid ; (iii) using a balloon catheter; and (iv) pulling back or lifting the electrode. Among them, artificial ascites has gained acceptance as a simple and effective technique for successful ablation.”
- 3.
“Complete ablation is the last key to a successful local treatment. The ablative margin surrounding the index tumor is an established prognostic factor for local tumor control. Local tumor progression can develop at the margin of an ablation zone if the ablative margin is inadequate compared with acceptable ablative margins. So that there is enough ablative margin, three factors should be considered before or during the ablation: (i) the tumor size with ‘mental’ 3-D configuration; (ii) the configuration and size of the RFA zone made by a specific electrode; and (iii) the direction of the electrode path related to the tumor configuration. We believe that a complete ablation depends on achieving a symmetric ablative margin rather than simply increasing the volume of the ablation zone. Regarding the tumor configuration, 3-D measurement of the index tumor must be done accurately. An effective direction for the electrode path depending on the expected RFA zone made by a specific electrode, should be selected. Finally, targeting of the intended site of the tumors (the location determined during the planning phase) must be performed accurately because position changes of the electrode become difficult due to a poor sonic window caused by microbubbles. Hence, some experience in image-guided liver biopsy is mandatory for those involved in a RFA procedure. To solve this problem, multiple electrodes can be used, either at monopolar or bipolar modes, for achieving the desired ablative margin. Another inevitable obstacle in achieving a complete ablation is the heat-sink effect caused by abutting large vessels of more than 3 mm in diameter. There are many options to deal with this technical challenge. One can choose a more aggressive posture using an intraoperative Pringle maneuver (only for metastatic deposits), or combined therapy with an ethanol injection or TACE for a perivascular tumor. At our institution, we recommend selective TACE rather RFA if more than 90° of the circumference of the central tumor abuts large vessels, especially at the hepatic hilum, because the possibility of an incomplete ablation and biliary complications are relatively high. We retrospectively assessed the morphologic pattern and exact site of local tumor progression after RFA in 86 patients who developed local tumor progression after RFA using internally-cooled electrodes. The most common pattern was the peripheral nodular type. The exact site of the local tumor progression was concordant with an insufficient ablative margin in 85%, a contiguous vessel in 42% and a subcapsular location in 50%. To sustain complete ablation for index tumor, regular imaging follow up is essential. Most local tumor progressions are usually detected at the 3–4 month interval imaging studies, and can be treated by additional RFA. If the local tumor progression is very small or very subtle on planning US, one can use CEUS-guided procedure.”
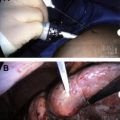
Less frequently, RFA is performed using an intraoperative or a laparoscopic approach. The first is mainly performed by surgeons in patients with multiple lesions unresectable by traditional surgery or in patients with extended hepatectomy and with a central lesion in the opposite lobe. The second is usually performed in patients with a difficult percutaneous approach. However, in some surgical centers intraoperative RFA is preferred also in cases treatable percutaneously. In a meta-analysis, Mulier and colleagues reported that RFA was performed percutaneously in 68% of cases, laparoscopically in 12%, or by laparotomy in 20%. In this meta-analysis, tumor-dependent factors with significantly minor local recurrences were small, neuroendocrine metastases, nonsubcapsular, and located away from large vessels, whereas physician-dependent favorable factors were surgical approach, vascular occlusion (in metastatic deposits), general anesthesia, a 1-cm intentional margin, and greater experience. Several factors may contribute to better results after a surgical approach. In particular, intraoperative US greatly improves spatial resolution, allows easy access to tumors located in the superior right lobe, and avoids damage by heating of adjacent organs, possible hemorrhage, or neoplastic seeding. We suggest the percutaneous route for patients who are too fragile to undergo laparotomy or for experienced operators confident enough to achieve a complete ablation. A paradoxic point of this review concerns the rate of local recurrence according to size and approach. It is not clear why in smaller lesions (<3 cm) the local recurrence rate was 16% after percutaneous approach versus 4% after surgical approach, whereas in medium-size lesions (3–5 cm, ie, those presenting fewer chances to be completely ablated), it was only 25% versus 21%, respectively.
Evaluation of therapeutic efficacy
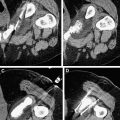
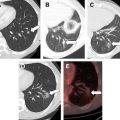
To evaluate the therapeutic response, that is to determine whether the tumor has become completely necrotic or whether areas of neoplastic tissue are still present, a combination of investigations and serum assays for tumor markers is used. They are the same as those adopted during initial staging and controls. Because there have been many investigations and some of them are comparable, we prefer to routinely use only contrast-enhanced US (CEUS) (with SonoVue [Bracco, Milan, Italy]) and spiral multislice CT with the triphasic technique (4–5 mL/s, 30, 60, and 120 seconds after the injection of contrast medium). Others imaging techniques (angiography, MR, positron emission tomography) or biopsy are performed only in rare cases, if there is a doubt whether the response is partial or complete. If the areas of viable tissue are very small, beyond the present powers of resolution, they are not recognizable on the images at the end of the treatment. However, they are easily identified at follow-up if they are shown as zones of enhancement at CT ( Fig. 1 ) or CEUS. The response is considered complete when CT and CEUS scans show the total disappearance of enhancement within the neoplastic tissue and when the same picture is confirmed at scans performed at successive controls. The absence of enhancement means the absence of blood flow because of necrotic and fibrotic modifications. Even with these characteristics, the necrotic area does not disappear and remains visible in place of the tumor even if reduced in size to different extents. CEUS is particularly useful during single-session treatment under general anesthesia in presence of large tumors because it permits evaluation before each possible additional insertion if there is persistence of any viable area. The following energy deposition can therefore be selectively performed in the enhanced tumoral tissue.
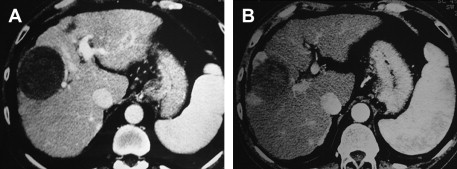
As tumor markers, we use α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP), which are often complementary. Nevertheless, their assay is useful only if they were abnormal before treatment. When the imaging techniques show a complete response not followed by normalization of AFP or DCP levels, it means that neoplastic tissue not detected or not yet detectable is growing elsewhere. Moreover, an increase in levels during follow-up always suggests a local recurrence or the appearance of new lesions.
We usually perform CEUS on the day after treatment, and contrast-enhanced CT if there is some doubt about ablation results. If CEUS does not present persistence of viable tissue, CT, CEUS, and serologic markers are performed every 4 months thereafter.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree