Introduction
Thyroid cancer, the most common endocrine malignancy, has a worldwide prevalence that continues to increase. It is estimated that 52,000 new cases were reported in the United States in 2019. The increasing use of ultrasound to detect small, nonpalpable, malignant thyroid nodules will cause this incidence to increase in the future. Thyroid cancer is a malignancy of the relatively young, mostly women in the fifth decade of their life at the time of diagnosis. Over 90% of the thyroid cancers are differentiated thyroid cancers (DTC), usually papillary or follicular carcinomas. Standard management of DTC involves total or subtotal thyroidectomy followed by the oral administration of radioactive iodine ( 131 I). The radioactive iodine will be absorbed and concentrated by benign and malignant thyroid cells because iodine is an inherent part of their normal metabolic activity. Because it is radioactive, the 131 I will eradicate postsurgical benign and/or malignant thyroid tissue remnants. The ablation also serves to diminish malignant recurrences, and facilitates postoperative surveillance via an improvement in the ability to monitor alterations in serum thyroglobulin. Furthermore, postoperative scanning with radioactive iodine (RAI) for recurrences will be more accurate if it does not have to compete with 131 I uptake by any postsurgical residual normal thyroid or neoplastic tissue. This therapeutic approach, thyroidectomy followed by 131 I ablation, has proven to be very effective and has a 10-year survival rate exceeding 80%.
Reassessment of 131 I Therapy
Unfortunately, the administration of RAI has been associated with adverse effects because of the tissue damage caused by radioactivity’s effect on other iodine-avid tissues, most frequently the salivary glands (SGs). Adverse SG reactions commonly include sialadenitis and less commonly taste aberration. Additionally, secondary SG adverse effects include increased occurrences of a primary SG malignancy and rarely, a transient facial nerve palsy. The concerns regarding these reactions originated when all DTC patients were subjected to 131 I therapy, mostly in the 100 mCi range. Such levels of radioactive iodine radiation are sufficient to cause secondary SG tissue damage with both subjective and objective symptomatology.
The relatively numerous adverse effects involving the SGs and other iodine-avid tissues have encouraged the reassessment of the use of RAI as a therapeutic tool. The papers of Schlumberger et al. and Mallick et al. have given impetus to the trend to utilize lower doses of 131 I for thyroid ablation. A meaningful decrease in the frequency and severity of complications, particularly the reported incidences of sialadenitis, has resulted. The transition to lower doses of radioactive iodine has been rapid and is supported by three observations. First, the published manuscripts of Schlumberger et al. and Mallick et al. have indicated that successful 131 I ablation can be achieved with 30 mCi and can be used to treat the low-risk patient. Objections to the finding of these authors have centered around the inadequate follow-up period (6 to 9 months) for their patients. Second, newly published American Thyroid Association guidelines state that the use of 131 I is not justifiable in all DTC patients. Although there is little controversy over its benefits in most patients, the use of 131 I has uncertain value in low-risk disease. Irradiation with 131 I can be avoided in those DTC patients who have no local tumor invasion, no metastases, and no aggressive histology associated with their existing neoplastic thyroid nodule. Third, the introduction of recombinant thyroid-stimulating hormone (rhTSH), as a substitute for the traditional preablation withdrawal of thyroid hormone and dietary iodine, has allowed the patient to remain euthyroid. , The period of hypothyroidism, with its reduction in quality of life caused by withdrawal of levothyroxine and dietary iodine, is no longer a concern. Renal clearance of serum 131 I occurs more rapidly in the euthyroid state than in a hypothyroid state. These three approaches, regarding radioactive iodine thyroid ablation, have succeeded in decreasing SG injury by decreasing the availability of toxic tissue doses of 131 I in the circulating serum. Despite a therapeutic trend to lower the RAI dose to a range of 30 to 50 mCi, treatment approaches using 75 to 100 mCi RAI for ablation are generally used. Ablation is considered successful when a scan reflects the absence of 131 I uptake by thyroid tissue and serum thyroglobulin is undetectable after TSH administration. Thyroid ablation has been reported to be more effectively achieved when 100 mCi RAI is administered.
Adverse Effects of 131 I
Regardless of the new norms in DTC care, secondary SG adverse effects will still be observed, albeit to a lesser extent and intensity. Adverse drug reactions are unintended responses resulting from the use of normal drug dosages that are being used for the therapy of a disease. The negative secondary consequences following the therapeutic use of RAI in thyroid cancer patients readily meet this definition. Prompt recognition of the symptomatology associated with these adverse reactions, most commonly SG injury induced by radioiodine, avoids misdiagnoses and leads to effective treatment.
SIALADENITIS
Within 24 to 48 hours after initial RAI therapy, SG pain and swelling often develop. Usually, the parotid glands are involved, often bilaterally. An inflammatory reaction is initiated by the 131 I radiation and leads to a vasculitis with endothelial wall injury and increased vascular permeability. , The escape of vascular constituents, fluid and cells with their cytokines, results in the patient’s clinical SG swelling and pain, which are almost always transient. This immediate problem has been reported to occur in as many as 50% of the recipients of this radioiodine ablation. Unfortunately, no data are available regarding low and high RAI dosages and their relationship to the frequency of this immediate post 131 I sialadenitis. This period of temporary SG swelling and pain coincides with the period of increased patient radioactivity that in the past required patient isolation.
Renal and salivary excretory activity rapidly come into play and function to lower the serum 131 I levels. Within a short time frame, the vascular endothelial wall reestablishes its normal permeability gradient. Abnormal extravasation terminates and the intraglandular elements of inflammation recede. Pain and swelling subside within a few days, and the patient usually enters a symptom-free period, which may be transient or permanent.
Following this early SG reaction, the first gland symptom that usually prompts a voluntary post-RAI therapy visit is a manifestation of 131 I-incited duct injury. It is obstructive in nature, and tends to occur months after an ablation with 75 mCi or more of radioiodine. The striated ducts of the SGs harbor the transport system for the 131 I. The sodium iodide symporter molecule, the transporter of the radioiodine, resides in the basolateral membrane of the striated duct. The SG ducts concentrate iodide by substituting iodide for Cl − as a substrate in the Na/K/Cl symporter molecule. The concentration of iodine in duct cells will reach a tissue-to-serum concentration gradient of 50:1. Calculations indicate that this extremely high concentration in the ducts is three to four times higher than what occurs in the parenchymal SG-secreting cells. Problems arise because the reproductive capacity for SG cells lies in the stem cells of the ductal system. The high radioactive iodine concentration in the duct damages the DNA of the stem cells that inhabit the ductal system. , Because the reproductive capacity for SG cells is possessed by these stem cells, delayed and decreased cell production and cell death with scarring are the inevitable consequences. Additionally, the irradiation’s effect on the cellular plasma membrane adds to acinar cell death.
All the SGs, particularly the major producers of saliva (two parotid glands [PGs] and two submandibular salivary glands [SMSGs]), are injured by the 131 I radioactivity. However, they are asymmetrically involved with any combination of 1 to 4 PGs/SMSGs demonstrating varying degrees of damage. Symptomatology is more often observed in the PGs than in the SMSGs. The relative resistance of the SMSGs as compared to the PGs has been attributed to the fact that the SMSG has a higher proportion of striated ducts than does the PG. These relatively more numerous striated ducts contain the iodide transport molecule that will allow for a higher rate of 131 I clearance through the SMSG than that which occurs in the PG. This rapid SMSG transit of the 131 I decreases the exposure time available for radiation injury. Additionally, SMSG secretion is uninterrupted even in unstimulated times. The persistent secretion acts to continually and consistently flush out radiation-containing saliva, thus decreasing the gland’s exposure to radiation. Simultaneously, the mucus element present in the SMSG secretion, but absent in PG secretions, serves as a protective asset against radiation.
Duct fibrosis with luminal stricturing reflects the end game of radiation-induced duct inflammation. The luminal passage is further narrowed by the development of intraluminal obstructive inflammatory exudates that act as blocking plugs ( Fig. 9.1 ). The duct constriction and inflammatory exudate combine to effectively limit the luminal passageway. Salivary flow is impeded, particularly during periods of increased salivary demand (eating), and SG pain and swelling follow as saliva backs up. In turn, the retained saliva adds to glandular inflammation, with swelling and pain accentuated by the inflammation caused by the physical presence of retained intraglandular saliva. As many as 44% of patients will develop symptoms of obstructive sialadenitis months after having been exposed to RAI ablation. Reported incidences of sialadenitis have varied because the investigations have not had a standardized universal design. Confusion exists because studies have involved different levels of 131 I dosages, varied means (objective/subjective) of evaluating the presence of adverse effects, and different follow-up periods.
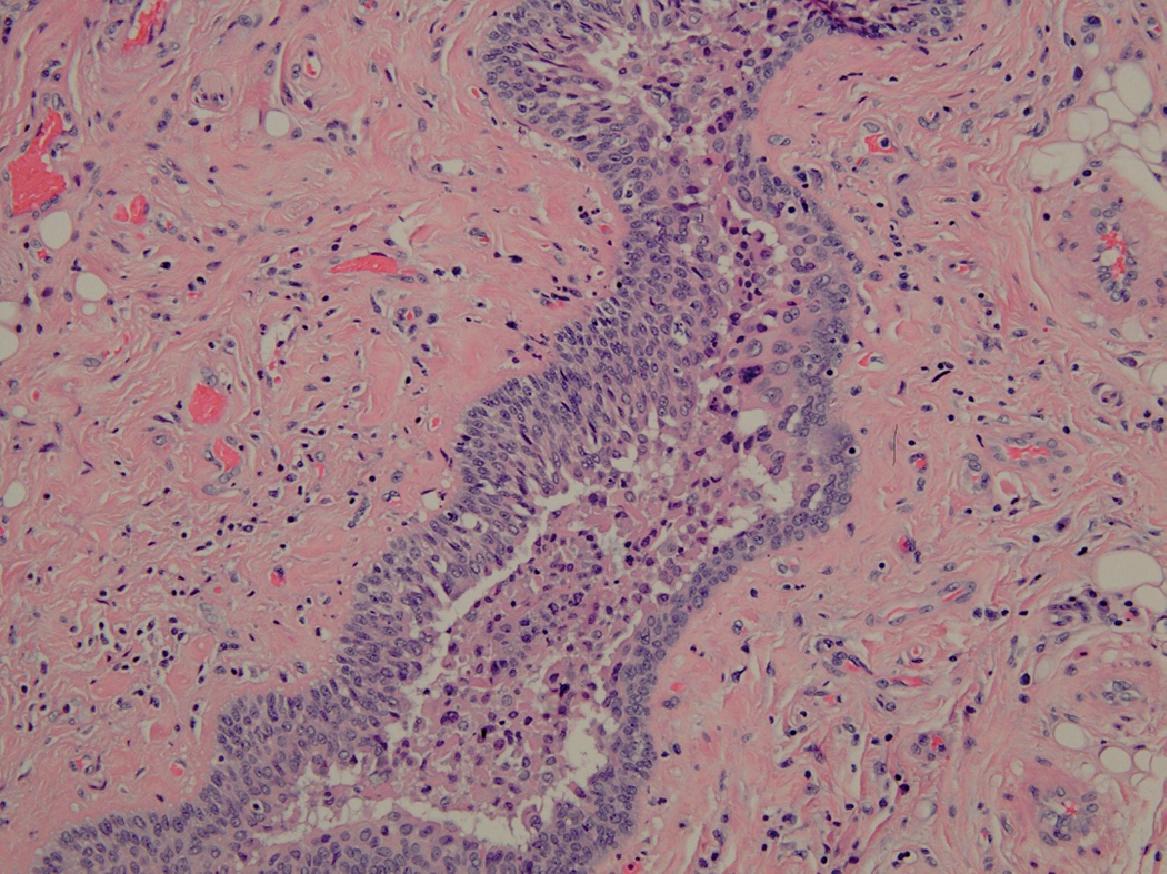
Although the glandular swellings are exacerbated when saliva is stimulated during meals, they partially subside after eating. The obstruction usually is not complete: a narrowed patent lumen will still be present. Therefore during unstimulated periods (between meals), glandular swelling and discomfort decrease as saliva slowly passes through the narrowed lumen. A diminution in SG swelling and pain is also made possible when the soft intraluminal plug formed by the inflammatory exudate is spontaneously extruded by the effects of increased retrograde hydrostatic salivary pressure. The patient will become aware of a salty taste because the damaged ducts have failed to adequately resorb sodium and chloride ions from the retained exiting saliva. Although some symptom amelioration now occurs, it may not be total. The SG inflammation initiated by the duct obstruction and the retained saliva can persist objectively and/or subjectively for varying periods, with remissions that can vary from weeks to months.
Because obstruction leads to salivary stagnation, an ascending secondary infection from the oral cavity is always a threat. The failure of duct flushing and the favorable media offered by stagnant retained saliva encourage bacterial colonization. The infectious process superimposed upon the already existing obstructed gland will cause a heightening of SG symptomatology that will include a pus-containing cloudy saliva. Continued SG exacerbations of pain and swelling from an infection-scarred and narrowed duct and a decreased salivary lavage can be expected.
Persistent obstructive symptomatology can lead to the development of a long-standing chronic sialadenitis. Diagnosis can readily be accomplished when the patient’s history of having received RAI is factored into the patient’s clinical complaints. Chronic obstructive sialadenitis has been reported to occur in as many as two-thirds of the patients who received 131 I ablation doses of 100 to 150 mCi. The damage to the SGs is in direct proportion to the administered 131 I dosage. , , In most patients, subjective symptom resolution eventually occurs, with only 5% of patients reporting subjective SG problems when seen 7 years after RAI treatment. Although some ductal strictures from the preexisting duct inflammation are present, the resulting narrowed lumen apparently is wide enough to accommodate the salivary demand and eliminate subjective complaints. Nevertheless, the sensitivity of a scintigraphic examination will usually reflect the presence of objective SG injury in the form of a decreased uptake with or without a secretory delay.
An initially high or repeated 131 I dose is required when tumor recurrence or metastases are present. Subjective and objective hyposalivation will then become evident if 131 I has been utilized in the range of 300 mCi. Apparently, the high level of radioactive iodine being absorbed by the SG is now sufficient to initiate a significant acinar cell loss. Lipid peroxidation, within the cell membrane from irradiation, precipitates cell death. , Further acinar cell loss results from DNA radiation damage in the progenitor and stem cells of the SG. , The decrease in acinar cell numbers results in a decreased salivary production. Gross SG parenchymal destruction with eradication of salivary production can be anticipated when total levels of 131 I reach 500 mCi. , Obstructive symptoms will not be present because parenchymal salivary production has been eliminated. A secondary ascending duct infection may now occur because of the absence of salivary lavage. This infectious inflammatory reaction is superimposed on the previously existing radiation-induced SG inflammation and accentuates it.
Objective authentication of gland function is most accurately determined via scintigraphy. A scintigram has the advantage of simultaneously evaluating in real time the activities of the two PGs and the two SMSGs. The radioisotope technetium 99m pertechnetate (TPT) is administered intravenously, circulates, and is picked up by the SGs. The TPT, a radioisotope of molybdenum, is considered safe because it only has a 6-hour half-life and it does not produce the destructive beta radiation associated with 131 I. The TPT emits a nondestructive gamma radiation that can be imaged by a gamma camera. The tracer is effectively concentrated by the SG. The SG is then stimulated with sour candy and its secretion-containing TPT is visualized by a gamma camera and graphed. The graph makes it possible to view the uptake and secretory abilities of individual glands in real time.
Because the damaging effect of 131 I on the SGs can best be illustrated via scintigraphy, four case outlines and their scintigraphs are presented to demonstrate the varied effects that different 131 I doses have on the SGs. Recombinant thyroid stimulating hormone was used in Cases 9.2 and 9.3.
Case Reports