Fig. 25.1
Fascial spaces of the retroperitoneum. A left adrenal gland, D duodenum, K kidney, P pancreas, SF splenic flexure of the colon
Anteriorly, the organ which is most commonly invaded is the stomach since the posterior parietal peritoneum overlying the pancreas is usually in contact with the visceral peritoneum covering the posterior wall of the stomach. Laterally, the spleen is frequently involved by tail lesions. The structures that share the pararenal space with the pancreas on its anteroinferior aspect include the fourth part of the duodenum, the splenic flexure of the colon more laterally, and the root of the mesocolon as noted above. For the surgeon the posterior relationships of the pancreas are the most important since the posterior resection margin is the most common site of a positive margin. Posteriorly and superiorly pancreatic tumors invade the splenic artery, the celiac artery, the common hepatic artery, and sometimes the origin of the left gastric artery. More posteriorly the superior mesenteric artery, aorta, and the confluence of the splenic and superior mesenteric veins may be involved. Pancreatic tumors also invade posteriorly through the anterior renal fascia to involve the adrenal and less commonly the kidney or the vasculature of these organs.
RAMPS attempts to maximize the chance of getting negative tangential margins by placing the resection plane behind the anterior renal fascia when the tumor has not penetrated the posterior capsule of the pancreas on preoperative CT scans and behind the adrenal gland and Gerota’s fascia when it has penetrated the posterior capsule [6] (Fig. 25.2). The goal is to add an extra margin of safety in resecting these tumors, which can spread microscopically beyond their radiographically visible or palpable margins. In each case the adrenal vein is the intraoperative guide to the position of the margin. In anterior RAMPS the posterior margin is formed by identifying the adrenal vein at its junction with the left renal vein and following its anterior surface retrograde in a right-to-left direction to the left adrenal gland. The posterior margin continues out on the surface of the adrenal and Gerota’s fascia. In posterior RAMPS, the adrenal vein is divided at its termination and elevated along with the adrenal to give the posterior margin. Not surprisingly larger tumors require a posterior RAMPS more commonly than small tumors.
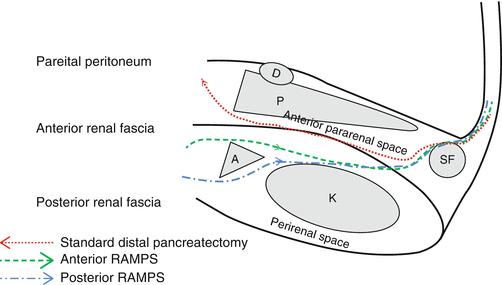

Fig. 25.2
Planes of posterior margin and direction of dissection in different types of distal pancreatectomies
25.2.2 Lymph Node Drainage (Fig. 25.3)
Both anatomical and pathological studies have been used to determine the propensity of a cancer to metastasize to specific lymph nodes. Pathological lymph node mapping studies use specimens obtained at surgery or autopsy to determine which lymph nodes are invaded in patients who have a particular tumor type. On the other hand, the anatomical approach uses dissection and injection of markers to identify the primary and secondary nodal drainage stations from particular organs. The aim of the RAMPS procedure is to perform a complete N1 node dissection and not resect N2 or N3 node levels. To do so the position of N1 nodes had to be defined, and we relied on anatomic studies of the lymphatic drainage of the body of the pancreas based on anatomic studies (Fig. 25.3 ) as summarized in the classic review by O’Morchoe [8].
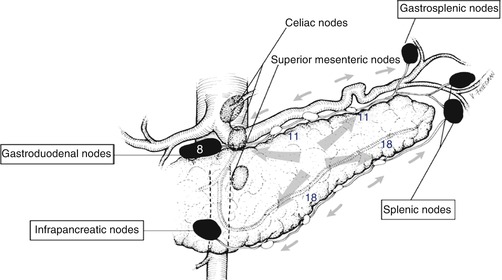

Fig. 25.3
Lymphatic drainage of body and tail of pancreas. The “ring” of nodes is named in boxes. The celiac and superior mesenteric nodes make up the “string” of nodes. The gastroduodenal node is node 8 in the Japanese classification, while the node chains on the superior and inferior borders are nodes 11 and 18. These nodes are most commonly involved in carcinoma of the body and tail of the pancreas
25.2.3 Summary of Anatomic Studies by O’Morchoe
The body and tail of the pancreas has four nearly equally sized quadrants. Lymphatic vessels traveling from the four quadrants connect to lymphatic vessels that lie on the superior and inferior borders of the gland (Fig. 25.3 ) [8]. Small lymph nodes are situated along these lymphatic vessels, and these are termed the suprapancreatic and infrapancreatic lymph nodes. These are node stations 11 and 18 in the Japanese Pancreas Society (JPS) classification. The lymphatic vessels on the superior and inferior borders of the left half of the body and tail drain to splenic nodes in the hilum of the spleen (JPS station 10) or to gastrosplenic nodes in the gastrosplenic omentum. These nodes lie in the gastrosplenic omentum along the short gastric arteries and correspond to JPS node station 4. O’Morchoe states that these nodes mainly receive lymph from the stomach but may also receive some lymph from the tail and left side of the body of the pancreas [8]. Lymphatic vessels coursing along the superior and inferior borders of the right half of the body drain to the gastroduodenal nodes (JPS station 8) and mesenteric nodes (JPS station 14c). These four sets of nodes form a ring of nodes [8] (Fig. 25.3). The efferent lymphatics from the ring of nodes drain into nodes that lie anterior to the aorta in relation to the celiac (JPS 9) and superior mesenteric arteries (JPS 14a), but these nodes, which may be thought of as a string of nodes, are not exclusively a N2 node group. Lymphatics from the central part of the pancreatic body enter these nodes directly without first entering a node on the ring [8]. Therefore, they should be considered as N1 as well as N2 nodes. As a result, operations designed to remove N1 nodes should resect both sets of N1 nodes, which we have colloquially referred to the “ring” and the “string” of nodes.
25.2.4 Summary of Pathological Studies
Kayahara et al. performed pathological mapping of nodes in cancer of the body and tail of the pancreas in 20 patients [10]. Three node groups were involved in more than 20% of patients − nodes along the superior and inferior borders of the pancreas (stations 11 and 18 in JPS system, respectively) and the gastroduodenal node (JPS node 8) (Fig. 25.3 ). These are all resected in the RAMPS operation. Fujita et al. [11] described the results of pathological lymph node mapping in 50 patients with adenocarcinoma of the body and tail of the pancreas. They identified a group of small lymph nodes attached to the pancreas, seen only on histological slides. These nodes were involved by the cancers in about 75% of patients. However, all other lymph node groups were involved very infrequently, usually in less than 10% of patients. The frequently involved nodes may correspond to the nodes that lie along the superior and inferior borders of the pancreas described in O’Morchoe’s study, [8] although those were grossly identifiable as nodes. Whether these nodes are exactly the same as those described by O’Morchoe, they are certainly removed by RAMPS. Kanda et al. studied 78 patients who had resection of the body and tail of the pancreas [12]. They noted that suprapancreatic nodes (21% of patients) and superior mesenteric nodes (10% of patients) were most commonly involved (JPS stations 11 and 14, respectively). Other stations were involved in less than 10%. The results of Fujita et al. and Kanda et al. are interesting, but it is unclear at present whether they apply to patients in Western countries since the incidence of cancer in lymph nodes seems lower than in American patients. Also as we will describe Japanese patients seem to have better differentiated tumors than American patients, a fact that also suggests that the disease may differ significantly in virulence in the two countries.
25.3 Technique of the RAMPS Procedure [2, 6, 7]
25.3.1 Preoperative Preparation
A recent computed tomogram is used to decide whether to perform an anterior or posterior RAMPS. When a rim of normal pancreas remains posterior to the tumor, the anterior RAMPS is chosen (Fig. 25.4). When the posterior margin of the tumor contacts or appears to break through the posterior capsule of the pancreas, the posterior RAMPS is selected (Fig. 25.5). The latter is more common in large tumors as might be expected. The tumor does not need to be seen to be touching or invading the adrenal for a posterior RAMPS to be selected. It needs only to be seen to have invaded posteriorly out of the pancreas. The principle is that the space between the back of the pancreas and the front of the adrenal is too thin to reliably attain negative margins when the tumor is present in the space. Of course in some instances when the tumor is very far to the left toward the hilum of the spleen, it is well away from the adrenal. In those cases the perinephric fat down to the level of the left kidney and occasionally the left kidney itself must be removed, and in some of these cases, the left adrenal may be spared. The operation in this respect is modeled around involvement of the left adrenal since it is by far the most common organ that requires resection other than the pancreas and the spleen. In our experience the left adrenal is removed in about 30% of patients.
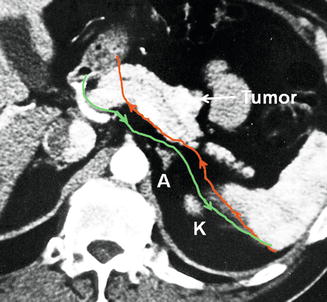
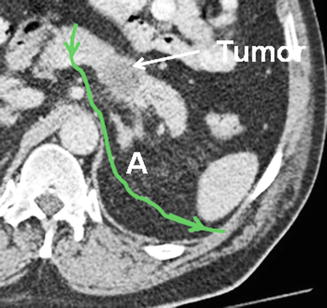

Fig. 25.4
Green line shows planned plane of posterior dissection as shown in preoperative computed tomogram in anterior RAMPS in which the tumor has not penetrated the posterior capsule of the pancreas. Note that the plane is on the anterior surface of the adrenal. Red line shows possible plane when standard distal pancreatectomy is performed without regard to the position of the anterior renal fascia. A left adrenal gland, K kidney

Fig. 25.5
Green line shows planned plane of posterior dissection as shown in preoperative computed tomogram in posterior RAMPS in which the tumor has penetrated the posterior capsule of the pancreas. A left adrenal gland
25.3.2 The Procedure
Staging laparoscopy is performed to detect intra-abdominal metastases, which contraindicate the procedure. In a study published in 2002, we found that 50% of laparoscopically staged patients had metastases [13] although with improved computed tomography techniques that figure is probably much lower today. A left upper quadrant “J” incision or “Mercedes Benz” incision with a longer left limb is used. The abdomen is again explored for evidence of metastases. The greater omentum is freed from the colon. The gastrosplenic ligament is divided taking the short gastric vessels close to the stomach in order to remove the gastrosplenic node group (JPS station 4). The lesser sac is entered much as in performing a Whipple procedure and the middle colic vein traced to the superior mesenteric vein. The neck of the pancreas is elevated off the superior mesenteric and portal veins. The right gastroepiploic vein may be sacrificed if necessary to display the superior mesenteric vein. A wide Kocher maneuver is performed and the anterior surface of the inferior vena cava is exposed. Then the left renal vein is exposed for several centimeters. The plane created on the left renal vein is behind the anterior renal fascia. This is quite useful later in the procedure when the anterior renal fascia has to be divided exposing the renal vein on the left side of the aorta.
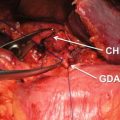
The lesser omentum is opened and the right gastric artery is divided. The proper hepatic artery is identified and followed proximally to display the common hepatic artery and gastroduodenal arteries. The gastroduodenal node (JPS node 8) is mobilized from above downward and left attached to the superior border of the pancreas, as the common hepatic artery is displayed. The anterior surface of the portal vein is exposed by retracting the gastroduodenal artery to the right. When the neck of the pancreas is less than 1 cm in thickness, it is divided using a stapler coated with collagen matrix sheets (“Peri-Strips” Baxter, Deerfield IL). When thicker than 1 cm, four stay sutures are placed in the neck of the pancreas, which is then divided with blended cutting cautery. The pancreatic duct is closed with a figure-of–eight 5-0 polypropylene suture (Prolene, Ethicon), and the stump of the pancreas is oversewn with several 2-0 silk full-thickness mattress sutures. Coagulating current is avoided for division of the pancreas because the resultant char may obscure the position of the pancreatic duct which is often of small diameter. Another factor taken into account is the shape of the pancreatic neck which is usually ovoid and of equal thickness in cross-section. However, occasionally the pancreatic neck is triangular in cross-section, and in these cases, stapling is less effective. A celiac node dissection is performed starting by incising the peritoneum over the crus of the diaphragm and progressing anteriorly and inferiorly from that point sequentially gathering lymph nodes, off the celiac artery and the origins of the left gastric and common hepatic and splenic arteries. Note that the origin of the splenic artery is identified as the nodes, and surrounding fat and fibrous tissue are cleared off the celiac artery and surrounding ganglia. The celiac ganglion is not resected. The splenic artery is occluded with a bulldog clamp and the common hepatic artery pulse is checked. The splenic artery is then divided between silk ties. It is our practice to tie and suture ligate the proximal part of the artery before dividing it. Occasionally, when the tumor is close to the origin of the splenic artery, the celiac and common hepatic arteries are occluded with vascular clamps; the splenic artery is cut almost flush with the celiac artery and oversewn with 5-0 polypropylene suture. In deep patients, usually men, the origin of the splenic artery may actually lie posterior to the pancreas, and it can be difficult to expose in that position until the neck of the pancreas and the termination of the splenic vein are divided. Also the origin of the left gastric artery may be involved and is then sacrificed. This is uncommon and usually occurs when the tumor has become attached to the lesser curvature of the stomach.
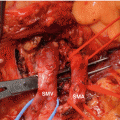
The splenic vein is isolated at its junction with the superior mesenteric vein and divided with a vascular stapler. If tumor invasion is present at this site, a resection of the superior mesenteric vein and/or portal vein is performed and repaired primarily or with a vein graft. The right border or the dissection is carried downward in the sagittal plane, dividing fat and fibrous tissue until the left side of the superior mesenteric artery is identified (Fig. 25.6). The artery is followed on its left side, superiorly and posteriorly, down toward the aorta. The lymph nodes anterior and to the left of the superior mesenteric artery are taken with this step.
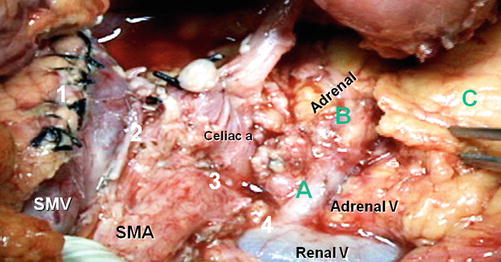

Fig. 25.6




Anterior RAMPS at completion of dissection. Numbers 1–4 show the four levels of the sagittal dissection 1 pancreatic neck, 2 splenic vein, 3 side of celiac and superior mesenteric artery, and 4 renal vein. (A – C) Shows the subsequent more coronal dissection. (A) Along the adrenal vein; (B) on the adrenal gland; and (C) along the surface of Gerota’s fascia
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree