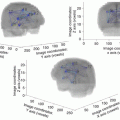
Fig. 26.1
Proton radiotherapy plan (left) compared to a photon plan with IMRT (right). The proton plan demonstrates decreased low dose spill (green and blue colorwash) compared to the IMRT plan
Greenberger et al., examined outcomes in 32 pediatric LGG patients who received proton radiotherapy. The GTV with a 3–5 mm expansion for PTV was treated to a mean dose of 52.2 GyRBE. PFS at 6 and 8 years was 89.7 and 82.8%, respectively. Overall, there was not a significant decline in neurocognitive status, but patients age < 7 and with significant dose to the left temporal lobe were more likely to suffer decline [31]. Dennis et al., performed a dosimetric exercise in LGG patients and demonstrated an estimated increased risk of secondary malignancy with IMRT compared to protons by twofold [32]. Shih et al., performed a cohort study of 20 adult patients treated with proton therapy for LGG (54 GyRBE in 30 fractions) and found a PFS rate of 85% at 3 years which dipped to 40% at 5 years. There was no overall decline in neurocognitive functioning or quality of life over a 5 year interval [33]. There are 2 currently accruing single arm phase II trials assessing endocrine and neurologic sequela in LGG patients treated with proton therapy [34, 35]. At present, proton radiotherapy is a reasonable option in young children where dose to the hippocampus and temporal lobe may be spared compared to IMRT.
26.8 Measuring Response to Radiotherapy
While robust response assessments tools have been validated in high grade glioma patients, standardization of response criteria in low grade glioma patients has been more difficult. This is due in part to the relative rarity of LGG compared to HGG and the variability of radiotherapy timing after definitive resection. Accurate assessment of response to radiotherapy is vital in order to distinguish between recurrence, radiation necrosis, or pseudoprogression. The Response Assessment in Neuro-Oncology (RANO) group published response assessment guidelines in LGG and defined progression by the following: (1) increase in enhancement or development of new lesions, (2) 25% or more increase in T2 or FLAIR abnormality from baseline in the presence of stable or increasing corticosteroid dose, (3) clinical deterioration in the absence of decreasing steroid dose or reasons not clearly attributable to the tumor. Radiographic response of the T2 or FLAIR abnormality compared to baseline imaging is the major differentiator between measuring complete response (no abnormality), partial response (≥50% reduction in area of the tumor), minor response (25–50% reduction) and stable dose (<25% reduction or <25% growth) [36].
Ducray et al., examined tumor response kinetics in 39 LGG patients after radiotherapy. Median tumor diameter (MTD) decrease was observed in 37 out of 39 patients and duration of MTD was 1.9 years. Patients whose tumors expressed 1p19q codeletion demonstrated a longer duration of MTD decrease (5.3 vs 1 years respectively). MTD was found to occur in two phases: an initial rapid decrease followed by a second more gradual phase of decrease. Patients with an initial rapid MTD decrease of over 7 mm/year had an inferior prognosis and shorter MTD which is likely due to more biologically aggressive disease [37].
Approximately 20% of LGG patients develop pseudoprogression after radiotherapy. Lin et al., found that presence of a 1p19q codeletion in radiated oligodendroglioma and oligoastrocytoma patients conferred a 10 fold decrease in the risk of developing pseudoprogression. This is in contrast to high grade glioma, where MGMT methylation is associated with an increased risk of pseudoprogression [38]. While the mechanism behind this is not entirely clear, pseudoprogression is thought to be possibly correlated to p53 expression, which is typically overexpressed in MGMT methylated high grade gliomas and underexpressed in 1p19q codeleted low grade gliomas [39].
26.9 Reirradiation for Recurrent Tumors
When recurrence is suspected, a biopsy is recommended when safe. In a report from Mayo Clinic, 51 previously irradiated LGG patients with suspicion of recurrence underwent biopsy of which 9% were found to either have radiation necrosis or radiation induced sarcoma. Approximately 2/3 of patients found to have recurrent glioma had transformation to high grade tumors [40]. Systemic therapy alone is a valid treatment option for recurrent tumor after radiotherapy, particularly if the interval from radiotherapy is short, the recurrence is near previously irradiated critical structures, or if the area of recurrence is very large. In chemotherapy naïve patients, salvage systemic therapy appears to be beneficial, particularly in 1p19q codeleted tumors [41]. However, the efficacy of salvage systemic therapy in patients who received TMZ or PCV initially is less clear [42].
Decisions regarding which patients are ideal for reirradiation should be made as part of a multidisciplinary discussion and take into account expected toxicity of treatment and life expectancy. To decrease the risk of radiation necrosis, often concurrent and adjuvant bevacizumab are used with reirradiation [43].
SRS and SRT are often utilized in previously irradiated patients due to the need to avoid large areas of high dose. In addition, concern for failure outside the gross tumor volume is reduced in patients with recurrent disease, thus justifying the need for reduced or no CTV margins. Ideal candidates for these techniques would include well localizable disease on imaging and low volume area of recurrence. The group from University of Pittsburgh reviewed their experience in treating recurrent grade II astrocytomas and oligodendrogliomas with single fraction radiosurgery. The astrocytoma population was treated to a median dose of 14 Gy and had a 5 year PFS of 54%. Predictors of improved PFS included tumor volume < 6 cc and doses ≥15 Gy [44]. The oligodendroglioma population was treated to a median dose of 14.5 Gy and had a 5 year PFS of 82%. Patients with 1p19q codeletion had improved PFS [45]. It is important to understand that these are small retrospective studies and only a subset of patients received prior irradiation.
Fogh et al., published results of 22 patients who received salvage reirradiation for LGG. The GTV (without a margin for PTV) was treated to a median dose of 35 Gy in daily fractions of 3.5 Gy. Clinical improvement was noted in 50% of patients [46]. This schema was also evaluated prospectively in high grade glioma patients as part of RTOG 1205, with final results pending [47].
Combs et al., examined 63 patients with recurrent low grade glioma who received reirradiation using fractionated SRT. Median dose was 36 Gy given in 2 Gy daily fractions prescribed to the tumor and a 1 cm margin. Median PFS was 12 months and OS was 24 months with no serious toxicities reported [48].
26.10 Brainstem Gliomas
Brainstem gliomas are more commonly diagnosed in the pediatric patients. High grade brainstem gliomas are often found within the pons and present as a diffuse infiltrative mass often involving large portions of the brainstem [49]. Lower grade lesions tend to present as more focal and dorsally exophytic [50]. Often brainstem LGG have an indolent clinical course followed by sudden onset of cranial nerve palsies. Due to the eloquence of the brainstem, definitive fractionated radiotherapy is the mainstay of treatment. The standard dose is 54 Gy delivered in 1.8–2 Gy fractions. Studies investigating altered fractionation including hyperfractionation with dose escalation and hypofractionation have not demonstrated a benefit over conventionally fractionated radiotherapy [51, 52]. GTV is optimally defined on T2 or FLAIR imaging with a 1–1.5 cm margin added to form CTV and an institutional margin for PTV. Combs et al. examined fractionated SRT in adults with brainstem glioma (median dose 54 Gy using 1.8 Gy per fraction). Eight percent of patients received FSRT for re-irradiation. CTV margin was not used. Treatment was well tolerated with a median OS of 81 months and median PFS of 52 months [53].
26.11 Future Directions and the Impact of Molecular Classification
Perhaps no other malignancy is currently undergoing a more radical change in classification than gliomas. Passaw et al. performed a molecular analysis of grade II-IV gliomas and found that TERT mutation (which encodes telomerase), IDH mutation, and 1p/19q codeletion were powerful predictors of survival. So called “triple positive” patients had the highest survival. On multivariate analysis, “triple negative” patients had a hazard ratio for death of 3.74, while TERT mutation alone conferred a hazard ratio of 11.74. Anaplastic histology conferred a more modest hazard ratio for death of 1.49, when compared to grade 2 tumors [54]. The Cancer Genome Atlas Research Network performed genomic analysis of grade II and III gliomas and found IDH, 1p/19q, and TP53 status to be more predictive of outcomes than grade. In fact, LGG histologies that were IDH wild type had molecular and clinical behavior patterns similar to glioblastoma [55]. This has enormous implication for therapy. RTOG 9402 randomized patients with grade 3 gliomas to either RT or PCV followed by RT. On initial report, no survival benefit was seen. However upon longer followup, there was a profound survival difference in the 1p/19q codeleted subgroup favoring PCV (14.7 vs 7.3 years respectively). These results mirror those of RTOG 9802 and underscore the similarities of behavior of grade 2 and grade 3 gliomas in subsets that harbor beneficial mutations [56]. Many have taken to referring to grade 2 and 3 gliomas with a favorable molecular profile as “lower grade gliomas.” Future trials may enroll so called “triple negative” or TERT mutated lower grade gliomas in trials evaluating glioblastoma patients, given that traditional therapies are proving less effective in this population. Conversely, patients whose molecular profile confers the longest disease free survival interval may be more suited to treatment deintensification. In regards to radiotherapy, this latter group of patients is the most likely to suffer long term neurocognitive effects of radiotherapy given their long life expectancy. These are potentially the ideal patients to receive proton radiotherapy. A randomized trial addressing the benefit of proton therapy in this group is currently under development through NRG Oncology.
It is important to understand that treatment stratification based on molecular profiling has not yet been reported prospectively. Examinations of predictive and prognostic capabilities of IDH, TERT, and TP53 have only been examined retrospectively or as an unplanned subgroup analysis of prospective studies. Thus, outside the auspices of a clinical trial, genomic classification should not be used to guide clinical decision-making.
Current understanding of low grade glioma is changing at a rapid rate. Perhaps the reason that the role of radiotherapy has been so poorly understood is that our understanding of the biology of this disease has been limited. It is imperative that the field of radiation oncology understand the new landscape of molecular classification in order to better tailor adjuvant therapies to patients.
References
1.
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW, Kleihues P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007;114(2):97–109.CrossRefPubMedPubMedCentral
2.
3.
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–55.CrossRefPubMedPubMedCentral
4.
Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, Coons S, Ricci P, Bullard D, Brown PD, Stelzer K. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016;374(14):1344–55.CrossRefPubMedPubMedCentral
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree