Fig. 7.1.
Axial CT view of an image-guided IMRT plan for cervical cancer. Image courtesy of C. Yashar.
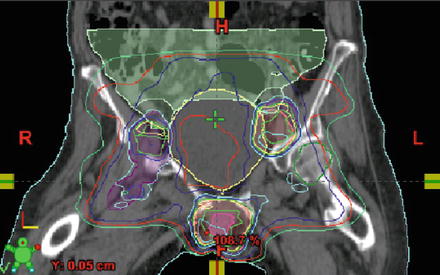
Fig. 7.2.
Coronal CT view of an image-guided IMRT plan for vulvar cancer. Image courtesy of C. Yashar.
No radioactive sources within the body.
Radiation is delivered without machinery touching the patient directly.
Specialized forms of external beam radiation therapy.
Stereotactic radiotherapy.
Uses a standard linear accelerator to deliver high-dose to precise locations in the body.
Stereotactic radiosurgery (SRS) delivers radiation to precise locations in the brain.
Stereotactic body radiotherapy (SBRT) delivers radiation to precise locations in the body.
Proton therapy.
Uses a beam of protons to deliver radiation to malignant tissue.
Main benefit: improves radiation localization and minimizes exit dose (which can minimize total dose to normal tissues, especially when tumor and normal tissues are juxtaposed).
Cyber Knife.
Radiation is delivered from a robotic arm and targets radiation at any body part from any direction using fiducial markers as guidance
Gamma Knife.
Utilizes cobalt and aims gamma radiation to precise locations in the brain for the treatment of brain tumors with the intent of delivering an ablative dose of radiation in one treatment session.
Local Irradiation (Brachytherapy)
Radiation emitted from natural isotopes (e.g., iridium, cesium) is predominantly used for interstitial radiation therapy, intracavitary radiation therapy, or brachytherapy [2].
What is brachytherapy?
Radiation is delivered to tissues from sources that are placed inside the body close to the tumor.
Intracavitary brachytherapy:
Radioactive source is placed directly in a body cavity (e.g., the vagina, Figs. 7.3, 7.4, and 7.5).

Fig. 7.3.
Applicator used for treating cancer of the cervix, endometrium, and vagina. Image courtesy of C. Yashar.
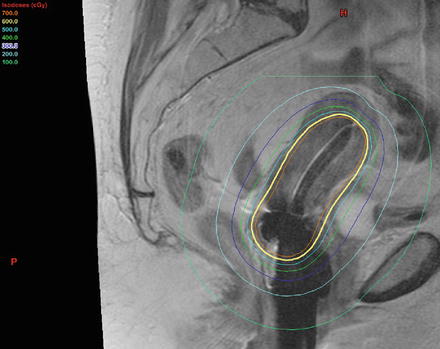
Fig. 7.4.
Sagittal MRI display with brachytherapy equipment in place. Colored lines represent distribution of dose around the applicator, with dose decreasing as a function of the inverse square law. Image courtesy of C. Yashar.
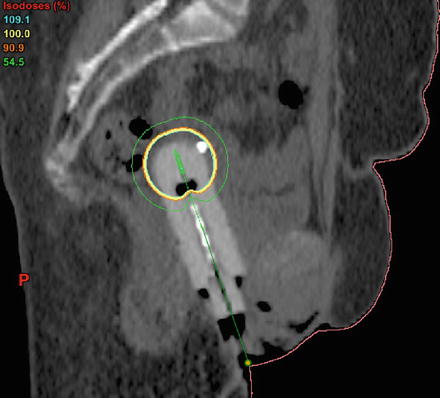
Fig. 7.5.
Vaginal cuff brachytherapy. Image courtesy of C. Yashar.
Interstitial radiation therapy:
Radioactive source is placed directly in the tumor bed or body tissue (e.g., the prostate) that isn’t a natural cavity.
Typically delivered at either a low-dose-rate (LDR) or high-dose-rate (HDR) system.
LDR systems require hospital admission such that the patient may stay in a shielded room and are less frequently used in the modern era of radiation oncology.
LDR systems deliver dose at a rate of around 50–120 cGy/h.
HDR systems are more frequently employed and deliver doses at 100 cGy/min.
HDR systems are typically employed on an outpatient basis.
Applicators that can be loaded with radioactive materials (primarily iridium) are used to administer intracavitary radiation.
Needles that can contain radioactive materials are used to deliver interstitial radiation.
Radiation Field Margins (for 3D CT-Based Planning) [6]
Postoperative therapy of cervical cancer and endometrial cancer.
Clinical Target Volume (CTV) Definition—identifies target that may contain microscopic spread of disease.
Common, external, and internal iliac lymph node regions, and the upper 3.0 cm of vagina and paravaginal soft tissue lateral to the vagina.
For patients with cervical cancer (or endometrial cancer with cervical stromal invasion), the CTV should include the presacral lymph node region.
Superior border of CTV: begin 7 mm below the L4-L5 interspace (although there is consideration with 2D planning of covering all the common nodes which may join to form the aorta/inferior vena cava more cephalad than the conventional 2D border of L4/L5).
Inferior border of CTV: extend to 3.0 cm below the upper extent of the vagina (defined by the vaginal marker) or to 1.0 cm above the inferior extent of the obturator foramen, whichever is lower.
Uniform 3D planning target volume expansion: 7 mm.
Radiation Field Margins (for Bony Anatomy-Based Planning)
Pelvic radiation Stage 1B–4A cervical cancer and endometrial cancer.
Superior border: L4-L5 (with 3D therapy, transition to confluence of the common iliac arteries and veins may be practiced, ~L3).
Lateral border 1.5 cm beyond lateral margin of bony pelvis.
Inferior border mid-point of obturator foramen (allows coverage of upper vagina) or 4 cm below vagina marker, whichever is lower.
Posterior border: coverage of at least S3 and with more advanced disease the sacral hollow.
Anterior border: Just anterior to symphysis pubis.
Para-aortic Radiation
Addition of para-aortic radiation to pelvic treatment requires that superior border be moved to body of L1 vertebra, with lateral borders of para-aortic filed encompassing the vertebral processes.
Anterior border of para-aortic fields is 2 cm anterior to anterior surface of vertebral bodies.
Posterior border is 2 cm posterior to anterior surface of vertebral bodies.
Inguinal Radiation
Anterior field
Superior border: line 2 cm superior and parallel to inguinal ligament.
Lateral border: vertical line parallel to midline at anterior superior ileac spine.
Inferior border: 8 cm inferior and parallel to inguinal ligament and 1 cm below most inferior portion of the vulva.
Medial border: 2 cm from midline bilaterally.
(Above leads to a pair of parallelograms).
Posterior field.
Superior border: mid-SI joint.
Lateral border: 2 cm lateral to widest portion of true bony pelvis.
Inferior border: mid-point of obturator foramen.
Brachytherapy Landmarks [7]
Point A: 2 cm above external OS and 2 cm lateral to midline (refers to uterus). Represents the parametria.
Point B: 3 cm lateral to point A, or 5 cm lateral to midline (should represent the pelvic sidewall).
The remainder of the chapter provides detail regarding specific radiation protocols used to treat the initial presentation of the most common gynecologic malignancies involving the endometrium, cervix, vagina, and vulva, as well as a brief overview of ovarian cancer, primary peritoneal carcinoma, fallopian tube carcinoma. A general overview of palliative treatment options will be provided at the end of the chapter.
Use of Radiation Therapy in the Most Common Gynecologic Malignancies
Endometrial Cancer
Most common gynecologic malignancy diagnosed in the USA [8].
Standard of care: up-front surgery (with consideration of lymph node dissection) serves to stage the cancer.
Low risk patients are typically not given a nodal dissection at the time of surgery and are often observed following surgery.
Vaginal brachytherapy: used for more deeply invasive lesions, higher-grade lesions, older patients, or patients with lymphovascular space invasion as isolated high risk factor.
Pelvic and paraaortic irradiation: reserved for the highest risk Stage I patients with multiple high risk factors, high risk Stage I patients without a nodal dissection, and advanced stage patients.
See Table 7.2 for a summary of the key research studies and clinical trials that comprise the basis for the above general treatment recommendations [9, 10, 14–17].
Study
FIGO stage (eligible patients)
Control group
Comparison group(s)
Overall survival (OS)
Recurrence risk
Toxicity
Aalders et al. [9]
Surgical Stage I (540)
Surgery plus brachytherapy (BT) alone
Surgery and BT plus EBRT
(1) No OS benefit for additional EBRT (90 % in BT-alone group vs. 87 % in BT + EBRT)
(2) Subset analysis: OS benefit for BT + EBRT in Stage IC Grade 3 patients (82 % vs. 72 %)
EBRT decreased risk of pelvic recurrence (1.9 % in EBRT group vs. 6.9 % in brachytherapy alone, p < 0.01)
Complications reported include: rectovaginal fistula, urethral stricture (in BT-alone group) and small-bowel obstruction, bladder necrosis (in the BT + EBRT group)
PORTEC-1, Creutzberg et al. [10]
Stage IB (G2–3) or IC (G1–2) (715), specifically no IC3 patients
Surgery alone
Surgery and postoperative EBRT
No survival benefit for additional EBRT (81 %) versus surgery alone (85 %, ns)
Locoregional failures greater in surgery-alone group (14 %) versus surgery + EBRT (4 %, p<0.001)
Treatment-related complications greater in radiotherapy group (25 %) versus surgery-alone group (6 %, p < 0.001); mostly grade 1 toxicity
GOG-99, Keys et al. [14]
Stage IB–II (392)
Surgery alone
Surgery and postoperative EBRT
No overall survival benefit for additional EBRT (92 % in EBRT group vs. 86 % in surgery-alone group, p = 0.5)
EBRT reduced risk of recurrence (12 % vs. 3 % in surgery-alone group, p < 0.01); Among HIR subset, 26 % in surgery alone group versus 6 % with additional EBRT
Significantly more hematologic, gastrointestinal, genitourinary, and cutaneous toxicities in EBRT group
PORTEC-2, Nout et al. [15]
Stage I–IIA with high-intermediate risk features (427)
Surgery plus EBRT
Surgery plus vaginal brachytherapy
No difference in survival, 85 % versus 80 % (ns)
No difference in vaginal recurrence, locoregional relapse or isolated pelvic recurrences
Acute grade 1–2 toxicity higher in EBRT group (54 % vs. 13 % in BT group, ss)
JGOG-2033, Susumu et al. [16]
Stage IC–IIIC (385)
Surgery plus EBRT
Surgery plus chemotherapy (cyclophosphamide, doxorubicin, and cisplatin)
(1) Low/intermediate risk patients: no survival difference
(2) Survival benefit in high-risk patients for chemotherapy (90 % vs. 74 % for EBRT group, p = 0.006)
(1) Low/intermediate risk patients: no progression-free survival benefit (PFS)
(2) PFS benefit in high-risk patients for chemotherapy (84 % vs. 66 % for EBRT group, p = 0.024)
No difference in toxicity between groups (1.6 % of EBRT group had grade 3–4 toxicity vs. 4.7 % of chemotherapy group)
Italy, Maggi et al. [17]
High-risk endometrial, Stage IC G3, II G3, and III (345)
Surgery plus EBRT
Surgery plus chemotherapy (cisplatin, doxorubicin, cyclophosphamide)
No overall survival difference (69 % in EBRT group vs. 66 % in chemotherapy group, ns)
No PFS difference (63 % in EBRT group vs. 63 % in chemotherapy group, ns)
EBRT associated with gastrointestinal and genitourinary side effects; Chemotherapy associated with hematologic, nausea, and vomiting
Key Research Studies in the Treatment of Early Stage (Stage I–II) Endometrial Carcinoma
Aalders et al. (1980)
Randomized controlled trial designed to study the benefit of additional pelvic external beam radiation therapy (EBRT) following surgery and vaginal brachytherapy in the treatment of Stage I endometrial carcinoma [9].
Five hundred and forty patients with Stage I endometrial carcinoma received a total abdominal hysterectomy and bilateral salpingo-oophorectomy (with no pelvic lymph node dissection) followed by postoperative vaginal cuff brachytherapy. Patients were then randomized to no further treatment versus additional treatment with EBRT to the draining pelvic lymphatics.
EBRT significantly reduced the risk of local recurrence (1.9 % vaginal and pelvic recurrence rate in EBRT group vs. 6.9 % recurrence rate in the no additional treatment group, p < 0.01).
EBRT group non-significantly developed more distant metastases than the no additional treatment group (9.9 % vs. 5.4 %, 0.10 > p > 0.05) [9].
There was no overall survival benefit for additional EBRT observed at 9 years (90 % in the control group vs. 87 % in the EBRT group).
Poor prognostic indicators identified: age >60 years, FIGO Stage IB (previously termed FIGO Stage IC), histologic Grade 3, and lymphovascular invasion [9].
A subset analysis revealed that only patients with poorly differentiated (grade 3) tumors, infiltrating more than half of the myometrial thickness, benefit from additional external beam radiation therapy (overall survival 82 % in the EBRT group vs. 72 % in the no additional treatment group) [9].
The Post Operative Radiation Therapy in Endometrial Carcinoma 1 (PORTEC-1) trial.
Randomized controlled trial designed to address the benefit of postoperative radiation therapy following initial surgery for endometrial carcinoma [10–13].
Seven hundred and fifteen patients with Stage IB (grade 2–3) or Stage IC (grade 1–2) received a total abdominal hysterectomy and bilateral salpingo-oophorectomy (with no pelvic lymph node dissection). Patients were then randomized to receive EBRT versus no further therapy.
Significant reduction in local recurrence with EBRT (5.8 % in the EBRT group vs. 15.5 % in the NAT group at 15 years, p < 0.001), but no overall survival benefit [13].
EBRT was more likely to be associated with adverse events, with up to 26 % of patients in the EBRT arm experiencing toxicity (mostly grade 1–2) compared to 4 % patients in the control arm [11], with side effects from the radiation therapy seen to persist at 15 years post-treatment [13].
Given the absence of a survival benefit for EBRT and the relatively high rate of toxicity, EBRT is recommended to only be given to patients determined to be at high risk of recurrence.
Risk factors: age >60 years, grade 3 lesions, deep myometrial invasion.
Patients with 2 of these 3 high risk features (high intermediate risk, HIR, patients) were seen to have a 20 % risk of locoregional recurrence without radiation therapy, which decreased to 5 % following EBRT [10].
Thus, after PORTEC-1, it was felt that there remained an indication for EBRT in HIR patients, but should be avoided in low-intermediate risk patients [13].
GOG-99 trial
Conducted to assess the benefit of postoperative radiation therapy versus no additional treatment following surgery for endometrial carcinoma on recurrence-free interval as the primary outcome [14].
In this study, 392 patients with intermediate or high-intermediate risk were randomized following total abdominal hysterectomy with bilateral salpingo-oophorectomy (with select patients receiving pelvic lymph node dissection) to postoperative radiation therapy versus no additional treatment.
Significantly lower recurrence rate in the EBRT treated group compared to the group receiving no additional treatment, which was especially pronounced in the high intermediate risk patient subset (6 % in the EBRT group vs. 26 % in the no additional treatment group at 2 years, p < 0.01) [14].
Conclusion: postoperative radiation therapy significantly decreases the risk of recurrence in early stage endometrial carcinoma, but should be limited to patients with high intermediate risk features [14].
PORTEC-2
Because most recurrences for limited-stage endometrial carcinoma following surgery occur in the vaginal cuff, PORTEC-2 was designed to compare the efficacy of vaginal brachytherapy with pelvic EBRT for preventing vaginal recurrence following hysterectomy [15].
In this study, 427 intermediate or high-risk endometrial carcinoma patients received total abdominal hysterectomy with bilateral saplingooophorectomy (and no lymph node dissection) and were then randomized to receiving either EBRT or vaginal brachytherapy.
At 5 years, vaginal brachytherapy is as effective as EBRT for preventing vaginal recurrence.
No difference in locoregional-relapse, isolated pelvic recurrence, distant metastases, or overall survival [15].
Vaginal brachytherapy was associated with significantly less acute grade 1–2 gastrointestinal toxicity than the EBRT group (13 % vs. 54 %).
Conclusion: vaginal brachytherapy should be used in place of EBRT as the standard-of-care adjunctive therapy for patients that fit PORTEC-2 criteria [15].
Chemotherapy: used for patients with more advanced disease, or higher-risk limited stage disease.
JGOG-2033 trial
Conducted to compare postoperative pelvic radiation with chemotherapy for patients with >50 % myometrial invasion (Stage IC–IV) [16].
In this trial, 385 patients were randomized following TAH/BSO or radical hysterectomy (with the majority of patients receiving pelvic lymph node dissection) to receive either pelvic radiation therapy (AP/PA field to 45–50 Gy) or 3 courses of cisplatin/doxorubicin/cyclophosphamide.
At 5 years, no survival differences between the groups (progression-free or overall) [16].
On subset analysis, there was no difference for low or intermediate risk patients.
In the high-risk group (defined as patients with Stage IC and age >70 years old, or patients with Stage IC, grade 3 disease, Stage II, or Stage IIIA patients), chemotherapy was associated with an overall survival benefit compared to radiation (89.7 % vs. 73.6 %, p < 0.01) [16].
Maggi et al. (2006)
Compared EBRT versus combined platinum-based chemotherapy following surgery for high-risk endometrial carcinoma [17].
Three hundred and forty-five patients with high-risk endometrial carcinoma (defined as Stage IC, grade 3, Stage IIC, grade III, with >50 % myometrial invasion, and Stage III patients) were randomized to receiving either EBRT (to 45–50 Gy) or 5 cycles of cisplatin/doxorubicin/cyclophosphamide chemotherapy.
At 5 years, there were no differences in overall-survival or progression-free survival between the groups [17].
The authors noted that there was a trend toward delayed local relapse with radiation therapy, and a trend for delayed progression to distant metastatic disease with chemotherapy, but these trends were not significant [17].
Key Research Studies in the Treatment of Locally Advanced (Stage III–IV) Endometrial Carcinoma
For patients with higher-stage endometrial carcinoma, surgery, chemotherapy, and radiation therapy are all vital treatment components.
Hogberg et al. presented the pooled results from two randomized studies (NSGO-EC-9501/EORTC-55991 and MaNGO ILIADE-III) designed to address the benefit of chemotherapy following surgery and radiation therapy for advanced endometrial carcinoma [18].
Five hundred and thirty-four patients with high-risk Stage I–III endometrial carcinoma patients received TAH/BSO were randomized to receive radiation therapy alone or sequential radiation therapy and chemotherapy.
Additional chemotherapy improves progression-free survival, and there was a trend to improving overall survival [18].
GOG 184 trial
Randomized patients with advanced endometrial carcinoma (Stage III or IV) treated with surgery and tumor-volume directed pelvic irradiation to receive either cisplatin and doxorubicin or cisplatin, doxorubicin, and paclitaxel chemotherapy.
No difference in recurrence-free survival between arms.
The addition of paclitaxel was associated with increased toxicity [19].
GOG 122
Randomized trial designed to compare whole-abdominal radiation versus chemotherapy in patients with Stage III–IV endometrial carcinoma and no greater than 2 cm of residual disease following hysterectomy [20].
Three hundred and ninety-six patients who received a TAH/BSO were then randomized to receive either whole abdominal radiation (AP/PA fields to 30 Gy with 15 Gy boost to lymph nodes) or 8 cycles of doxorubicin and cisplatin chemotherapy.
At 5 years, significant improvement in overall survival for the chemotherapy group (55 %) compared to the group receiving abdominal radiation therapy (42 %), however with greater acute toxicity observed in the chemotherapy arm [20].
Approximately half of the patients in both arms experienced recurrence; patients in the chemotherapy arm tended to have higher rates of pelvic recurrence, whereas patients in the chemotherapy arm had fewer distant recurrences [20].
The whole abdominal radiation dose was relatively low with an outdated administration compared to techniques employed today.
Sandwich trials: administered adjuvant radiation therapy “sandwiched” between courses of chemotherapy.
Einstein et al. presented the results from a phase II prospective study designed to assess the tolerability of sequential chemotherapy with radiation therapy for advanced endometrial carcinoma [21]. Following surgery, patients were given a sequence of paclitaxel, radiation therapy, and carboplatin.
The treatment was well-tolerated, and the authors reported overall survival of 6.3 years for Stage I/II, 3.0 years for stage III/IV [21].
Secord et al. [22] presented the results of a multicenter retrospective analysis of patients with Stage III and IV endometrial carcinoma to assess the whether there was benefit for a particular sequencing of chemotherapy and radiation following surgery.
“Sandwich” chemotherapy-radiation-chemotherapy (CRC) was associated with improved survival compared to chemotherapy followed by radiation (CR) and radiation followed by chemotherapy (RC) [22].
Ongoing trials
GOG-0249
Designed to assess whether vaginal cuff brachytherapy followed by 3 cycles of chemotherapy (paclitaxel and carboplatin) increases recurrence-free survival compared to EBRT in patients with Stage I–IIA endometrial carcinoma with high-intermediate risk factors.
PORTEC-3
Designed to compare EBRT alone versus concurrent cisplatin-EBRT followed by adjuvant chemotherapy (paclitaxel and carboplatin) in high risk stage I–III patients.
GOG 0258
Addresses the benefit for concurrent cisplatin and tumor-volume directed irradiation followed by carboplatin and paclitaxel versus carboplatin and paclitaxel alone for advanced endometrial carcinoma patients.
Cervical Cancer
The third most common gynecologic malignancy diagnosed in the USA, following endometrial and ovarian cancer.
Formerly the most common cause of cancer-related mortality in the USA, however mortality from cervical cancer has decreased dramatically as a result of improved access to Papanicolaou smear screening programs [23].
Worldwide, however, cervical cancer remains the second most common cause of cancer-related mortality.
Treatment of Microinvasive (Stage IA) Cervical Cancer
The current primary treatment of Stage IA1 cervical cancer with no lymphovascular space invasion is cervical conization.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree