Author, year of publication
Study design
# of IHC pts
Tumor size (cm3)
Mode of RT delivery
Total dose (Gy)
# Fx
One-year LC
One-year OS
Toxicity
Liu et al. [40]
Retrospective
6
8.8 (0.2–222.4)
3D-conformal SBRT
20–50
3–5
93%a
81%a
None for IHC pts
Ibarra et al. [41]
Retrospective
11
80.2 (30.6–818.5)
SBRT, Cyberknife, or linac-based
22–50
1–10
50%
45%
7 with grade 3 toxicities
Lanciano et al. [42]
Retrospective
4
60.9 (2.29–316)
SBRT, Cyberknife
36–60
3
92%a
73%a
None for IHC pts
Barney et al. [43]
Retrospective
6
16–412.4
IMRT or 3D-conformal SBRT
55 (45–60)
3 or 5
100%
73%
1 w/Gr. 3 biliary stenosis; 1 w/Gr. 5 liver failurea
Dewas et al. [44]
Retrospective
6
63 (36–112)
SBRT, Cyberknife
45 (29–45)
3 or 4
100%
NR%
NR
Goyal et al. [45]
Retrospective
3
384 (80–818)
SBRT, Cyberknife
34 (24–45)
1–3
82% at 8 mos
NR
None
Goodman et al. [46]
Phase I
5
32 (0.8–146.6)
SBRT, Cyberknife
18–30
1
77%a
71.4%b
None for IHC pts
Kopek et al. [47]
Prospective
1
32 (9–205)
SBRT, linac-based
45
3
84%a
Median 10.6 mosa
21 w/Gr 3 elevation in liver enzymes, 6 w/GI bleeda
Tse et al. [39]
Phase I
10
172 (10–465)
SBRT, linac-based
36 (24–54)
6
65%
58%
2 w/transient biliary obstruction, 2 w/decline to CP B
Wulf et al. [48]
Retrospective
1
53 (9–516)
SBRT, linac-based
26–37.5
1–3
100%
60%
No grade 3
Herfarth et al. [49]
Phase I/II
3
10
SBRT, linac-based
14–26
1
71%c
72%c
None
Blomgren et al. [34]
Retrospective
1
67
SBRT, microtron, or linac-based
63
3
14 mos median
11 mos median
NR
Tao et al. [27]
Retrospective
79
198 (12–966)
3D-conformal, IMRT, or proton
58.05 (35–100)
28 (15–30)
81% at 1 yr
45% at 2 yrs
87% at 1 yr
61% at 2 yrs
7 w/biliary stenosis requiring stentd
Hong et al. [50]
Phase II
39
133.7 (3.7–599.7)
Proton
58 (15–67.5)
15
94.1% at 2 yrs
69.7% at 1 yr
46.5% at 2 yrs
3 w/Gr 3 liver failure, hyperbilirubinemia, gastric ulcer
16.3.4 Charged Particle Therapy
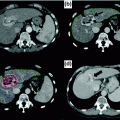
Charged particle therapy, including protons and carbon ions, represents a potential technique for increasing the dose to a tumor while minimizing damage to the surrounding hepatic parenchyma. Protons have a distinct physical advantage over standard photon-based radiation. Photons deposit energy along the beam path beyond the tumor; this exit dose often leads to unwanted radiation exposure to uninvolved hepatic parenchyma, thereby increasing the risk of RILD as it is mediated by the dose delivered and volume of liver irradiated [23, 51]. In contrast, protons have minimal exit dose, which provides a theoretical clinical benefit by allowing escalation to tumoricidal doses without compromising normal tissue. There are no randomized data on the use of charged particle therapy versus photon therapy for HCC or IHC. This modality, however, has been used effectively in the treatment of individual patients with IHC, often with dramatic shrinkage of the primary lesion (Fig. 16.1).
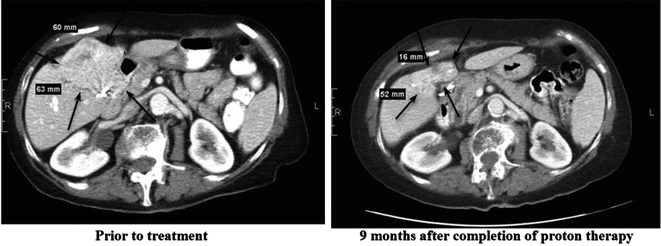

Fig. 16.1
Axial CT slices of an intrahepatic cholangiocarcinoma lesion before and after completion of proton therapy
Recently, a multi-institutional Phase II study was completed utilizing high-dose, hypofractionated proton beam therapy for localized, unresectable HCC and IHC [50]. Of the 83 evaluable patients, 44 had HCC and 39 had IHC; almost 90% of the IHC patients had no evidence of cirrhosis. For IHC patients, 34 (87%) had one lesion, three (8%) had two lesions, and two (5%) had three lesions. The median dose delivered was 58.0 GyE in 15 fractions. The average dose received by liver tissue not involved by tumor for all patients was 21.4 GyE (range 3.2–29.5 GyE). In the entire cohort, only four patients experienced at least one grade 3 treatment-related toxicity. One of the 44 HCC patients developed grade 3 thrombocytopenia; of the 39 IHC patients, one developed liver failure and ascites, one developed a stomach ulcer, and one was found to have hyperbilirubinemia. Three of the 83 patients (3.6%) had worsening Child-Turcotte-Pugh (CTP) score: two patients from A to B at three months and one patient from A to B at six months. There were no grade 4 or 5 treatment-related toxicities. In terms of efficacy, only four patients developed local progression within two years of follow-up; two-year local control rate was 94% for patients with either HCC or IHC; however, recurrence beyond two years only occured in patients with IHC, specifically in four additional patients. Notably, all patients who experienced local progression had received less than 60 GyE. For patients with IHC, the median progression-free survival was 8.4 months, median overall survival was 22.5 months, and two-year overall survival was 46.5%. The impressive results of this study, especially when compared with historical data using conventionally fractionated RT for IHC, have recognized high-dose, hypofractionated proton beam therapy as an attractive modality for inoperable intrahepatic tumors, especially those that are too large for the extreme hypofractionation associated with SBRT (Table 16.1).
16.4 Future Directions
Given the encouraging results seen with the addition of radiation therapy for localized, unresectable IHC, the NRG Oncology Group has developed a Phase III trial of gemcitabine and cisplatin with or without liver-directed radiotherapy to determine the impact of radiotherapy on outcomes in patients receiving optimal systemic therapy. Patients will receive three cycles of gemcitabine and cisplatin, followed by restaging and stratification based on tumor size (≤6 cm vs. >6 cm) and the presence or absence of satellite lesions. Patients will then be randomized to liver-directed radiotherapy and additional gemcitabine and cisplatin versus gemcitabine and cisplatin alone (Fig. 16.2). Selection of the prescription dose is to be based on the mean liver dose.
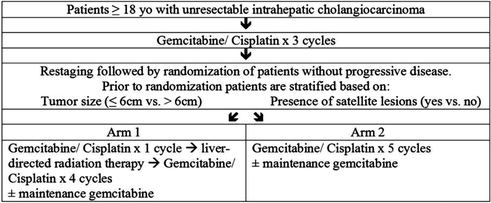

Fig. 16.2
Schema for NRG GI001 trial
16.5 Neoadjuvant Therapy with Transplantation for Hilar Cholangiocarcinoma
Cholangiocarcinoma arising at the hepatic duct bifurcation (i.e., hilar cholangiocarcinoma) characteristically arises in patients with primary sclerosing cholangitis (PSC). These patients, similar to those with severe cirrhosis, are usually not candidates for the extensive resection that would be needed to obtain negative margins. Despite the rare incidence of cholangiocarcinoma overall, patients with PSC are at significantly higher risk with incidence reported from 4 to 20%, and majority are hilar (i.e., Klatskin tumor) [52, 53]. Liver transplantation was studied as an alternative for those patients with localized disease who were not candidates for extensive resection. Initial outcomes were poor due to high incidence of locoregional dissemination and recurrence [54, 55]. Due to the potentially long waiting time for organ transplantation, neoadjuvant therapy with radiation and concurrent chemotherapy was proposed in order to obtain local control and decrease risk of regional recurrence following transplant [55, 56]. The University of Nebraska and the Mayo Clinic have demonstrated that excellent survival can be achieved for highly selected patients with early stage hilar cholangiocarcinoma treated with aggressive neoadjuvant therapy leading to liver transplantation.
The University of Nebraska initially studied this multimodal technique; seventeen patients with hilar cholangiocarcinoma, all presenting with obstructive cholangitis, were treated between 1987 and 2000 with chemotherapy (daily 5-FU 300 mg/m2) and intraluminal bile duct brachytherapy (iridium-192 6000 cGy delivered over 55–60 h) while awaiting liver transplantation. Patients were only eligible if maximal tumor dimension was 2 cm without radiographic extrahepatic disease or intra/extrahepatic metastasis. The basis for these guidelines was the fact that the iridium wires used for radiotherapy had a penetration of 1 cm; therefore, tumors greater than 2 cm in diameter may not achieve optimal dose at the periphery. Notably, nine patients had PSC and/or ulcerative colitis, and three patients had decompensated cirrhosis. The most significant complication between chemoradiation and surgery was recurrent episodes of cholangitis. Eleven patients were free of complications or tumor progression precluding surgery and underwent transplantation (median waiting time 87 days, range 15–792). The median survival was 25 months for the patients who underwent liver transplantation; five of these patients (45%) remained free of tumor recurrence 2.8–14.5 years after transplant [58].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree