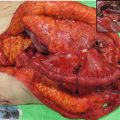
Fig. 13.1
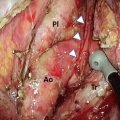
Example 3D treatment planning for a cT1bN0 middle thoracic esophagus tumor. (a) Endoscopic insertion of clips in the esophageal wall near the proximal and distal end of the primary tumor. (b) Target volume of local radiotherapy planning. Clips (blue), GTV of primary tumor (red), CTV of primary tumor (pink), GTV plus 2 cm margin proximally and distally along the length of the esophagus, PTV (orange)
Clinical Target Volume (CTV)
CTVp is defined as the GTVp with 2–4 cm expansion proximally and distally along the length of the esophagus. The intent is to extend the margin along the length of the esophagus to provide a margin for coverage of submucosal extension of the tumor. One pathological analysis of 34 surgical specimens of ESCC showed the mean microscopic spread beyond the gross tumor was 10.5 ± 13.5 mm proximally and 10.6 ± 8.1 mm distally, and placement of a 3 cm margin proximally and distally on the primary tumor would cover microscopic disease extension in 94 % of cases [11].
CTVn is defined as the GTVn with 0–0.5 cm margin in all directions.
The regional lymph nodes are defined as CTV subclinical (CTVs) for each primary site in the treatment of elective nodal irradiation. Several pathological analyses of surgical specimens of ESCC reported that the rate of positive lymph nodes per number of cases was 47–70 % and patterns of involved nodal spread were different from each primary site [12–14] (Fig. 13.2). Retrospective analysis from Japan showed that elective nodal irradiation was effective for regional lymph node failure [15]. Guidelines 2012 for the treatment of esophageal cancer in Japan shows the inclusion of regional lymph nodes in CTVs for each primary site (Table 13.1) (Fig. 13.3a–d). Typically, the regional lymph nodes include bilateral supraclavicular fossae, superior mediastinal, and subcarinal lymph nodes for carcinoma of the cervical esophagus and upper thoracic esophagus (Fig. 13.4a). Mid-jugular lymph nodes are also included for carcinoma of the cervical esophagus. And the regional lymph nodes include superior mediastinal, subcarinal, middle mediastinal, lower mediastinal, and perigastric lymph nodes for carcinoma of the middle or lower thoracic esophagus (Fig. 13.4b). Celiac axis lymph nodes are also included for carcinoma of the lower thoracic esophagus. There is no consensus about inclusion of regional lymph nodes in CTVs for carcinoma of the middle thoracic esophagus.
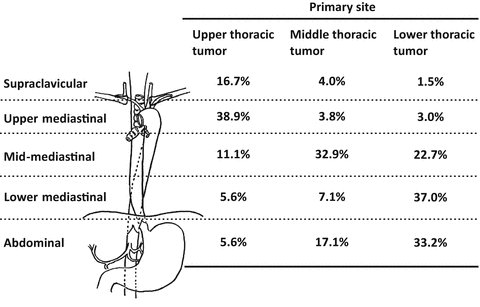

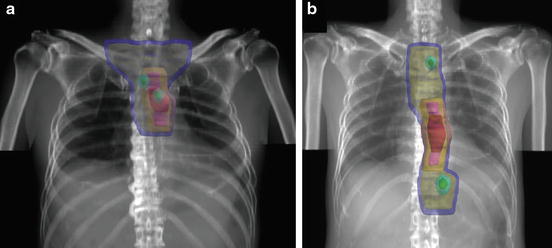

Fig. 13.2
Location and frequency of nodal involvement (%) by ESCC according to the primary site (From Huang et al. [14])
Table 13.1
Regional lymph nodes defined as CTVs for each primary site
Primary site | Regional lymph nodes |
|---|---|
Cervical esophagus | Mid-jugular lymph nodes, supraclavicular lymph nodes, superior mediastinal lymph nodes, subcarinal lymph nodes |
Upper thoracic esophagus | Supraclavicular lymph nodes, superior mediastinal lymph nodes, subcarinal lymph nodes |
Middle thoracic esophagus | a. Supraclavicular lymph nodes, superior mediastinal lymph nodes, subcarinal lymph nodes, middle mediastinal lymph nodes, lower mediastinal lymph nodes, perigastric lymph nodes |
b. Superior mediastinal lymph nodes, subcarinal lymph nodes, middle mediastinal lymph nodes, lower mediastinal lymph nodes, perigastric lymph nodes | |
Lower thoracic esophagus | Superior mediastinal lymph nodes, middle mediastinal lymph nodes, lower mediastinal lymph nodes, perigastric lymph nodes, celiac lymph nodes |

Fig. 13.3
Example of target volume delineation of CTV of elective nodal region. CTVs (yellow), PTVs (blue)

Fig. 13.4
Examples of target volume with elective nodal region in the 3D treatment planning for cT3N1 thoracic esophagus tumor. (a) For cancer of the upper thoracic esophagus. (b) For cancer of the middle or lower thoracic esophagus. GTV of primary tumor (red), GTV of metastatic lymph nodes (green), CTV of primary tumor (pink), CTV of elective nodal region (yellow), initial PTV (blue), boost PTV (orange and cyan)
Planning Target Volume (PTV)
PTV is defined as CTV with 1–2 cm margin in craniocaudal direction and 0.5–1 cm margin in the lateral direction to account for respiratory organ motion and daily setup error. Report of evaluating the respiratory motion of distal esophageal tumor using 4D-CT showed that a radical margin of 0.8 cm and axial margin of ±1.8 cm would provide tumor motion coverage for 95 % of the cases [16].
13.2.2.2 Field Design
In the treatment of target to the primary tumor and involved lymph nodes only, beam arrangement in 3D-CRT uses a multi-field technique such as a three- to six-field arrangement (Fig. 13.1b). By contrast in the treatment including the elective nodal irradiation, anteroposterior (AP)/posteroanterior (PA) fields are used up to 40–45 Gy followed by off-cord boost fields. For cervical esophageal tumor, right anterior oblique (RAO) and left anterior oblique (LAO) with wedged pairs are usually used as off-cord boost fields. For upper, middle, and lower esophageal tumor, RAO and left posterior oblique (LPO) are usually used as off-cord boost fields. At the beginning of initial treatment for a middle or lower thoracic esophagus tumor, a multi-field technique such as a four-field arrangement (AP/PA/RAO/LPO) is recommended considering the cardiac toxicity (Fig. 13.5). However, it is necessary to minimize the volume of irradiated lung (beam weight; AP/PA ≫ obliques) as to the lung toxicity. In the case of existence of hot spot such as >110 % of the prescribed radiation dose, field-in-field technique is considered to improve the conformity of the dose distribution. More recently, intensity-modulated radiotherapy (IMRT) has been considered, particularly cervical lesions. IMRT can further improve the conformity of the dose distribution by sparing the adjacent normal strictures such as spinal cord to help meet dose constraints (Fig. 13.6). Diametric comparisons of IMRT versus 3D conformal therapy in cervical esophageal cancer have demonstrated superior target volume coverage and conformality with decreased normal-tissue dose [17]. A potential disadvantage of IMRT is the possibility of delivering low doses of radiation therapy to normal-tissue areas. The influence of this on toxicity (low-dose pulmonary irradiation and development of lung toxicity) remains uncertain.
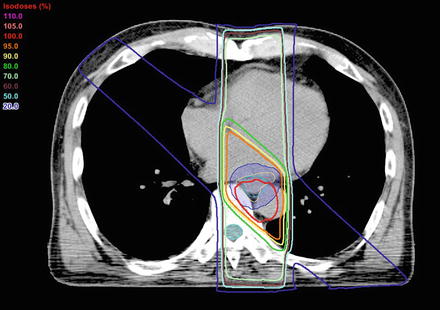
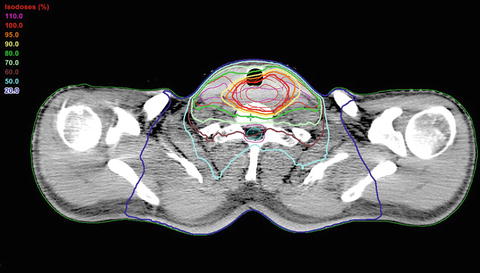

Fig. 13.5
Example of dose distribution treated with a four-field technique for a middle thoracic esophagus tumor (beam weights arrangement of 180 cGy per fraction; anterior 60 cGy, posterior 70 cGy, obliques 25 cGy). Daily heart dose: <80 % of the prescribed dose, Daily lung dose: <30 % of the prescribed dose

Fig. 13.6
Dose distribution of IMRT plan for a cervical esophagus tumor
13.2.2.3 Dose and Fractionation
Conventional daily dosing at 1.8–2.0 Gy fraction is standard. In the treatment of radiotherapy alone, 60–70 Gy at 1.8–2 Gy per fraction is standard radiation dose. In the treatment of chemoradiotherapy, based on the result of a randomized trial intergroup (INT) 0123 demonstrated that no significant difference in overall survival and local/regional control between the 50.4 Gy arm and the 64.8 Gy arm among patients (85 % SCC) treated with concurrent 5-FU and cisplatin chemotherapy for nonsurgical therapy [18], standard dose of radiotherapy for esophageal cancer is usually 50–50.4 Gy at 1.8–2 Gy per fraction in the definitive setting. In addition pattern care of study reported that median total dose of external radiotherapy was 60 Gy for definitive chemoradiotherapy patients in Japan [19]. In the neoadjuvant setting, 40–50.4 Gy at 1.8–2 Gy per fraction is the standard radiation dose.
13.2.2.4 Dose Constraints
In radiotherapy treatment planning of esophageal cancer, normal-tissue tolerance should always be considered. Accurate delineation of adjacent organs, including the lungs, spinal cord, heart, kidneys, and liver, is important. And it is necessary to evaluate the dose-volume histogram (DVH) analyses for each organ (Fig. 13.7). Max dose of the spinal cord is generally limited to 45 Gy using 1.8 Gy fractions. Several studies have demonstrated that dosimetric parameters derived from DVH are associated with organ toxicity after treatment of esophageal cancer [20–26]. In the treatment of esophageal cancer using neoadjuvant regimen of 45 Gy with concurrent chemoradiotherapy, a lung V10 (a percentage of lung volume receiving at least 10 Gy) of 40 % or greater, and a V15 of 30 % or greater, was shown to be predictive of significantly greater pulmonary complications (pneumonia and acute respiratory distress syndrome [ARDS]) [20]. Investigators from United States reported that the volume of the lung spared from doses of 5 Gy or higher (VS5) was the factor most strongly associated with postoperative pulmonary complications (pneumonia and ARDS) for esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery [21]. In the treatment of esophageal cancer using definitive regimen of 60 Gy with concurrent chemoradiotherapy, investigators from Japan reported that the optimal V20 threshold to predict symptomatic radiation pneumonitis (grade 2) was 30.5 % [22]. Konski and colleagues proposed thresholds for symptomatic cardiac toxicities (pericardial effusion, myocardial infarction, and sick sinus syndrome) for whole-heart V20 of 70 %, V30 of 65 %, and V40 of 60 % [23]. Wei and colleagues performed an analysis of pericardial effusion risk from DVH parameters among patients treated with definitive chemoradiotherapy [24]. Their data showed that the risk of pericardial effusion increased significantly with a mean pericardial dose of 26.1 Gy (p = 0.002) and pericardium V30 greater than 46 % (p = 0.001). Fukada and colleagues reported that mean pericardial doses of 36.5 Gy and V45 of 58 % were selected as optimal cutoff values for predicting symptomatic pericardial effusion [25]. For lower esophageal cancers, it is recommended that mean liver dose should be limited to less than 28 Gy, and mean dose of bilateral whole kidneys should be limited to less than 15–18 Gy [27].
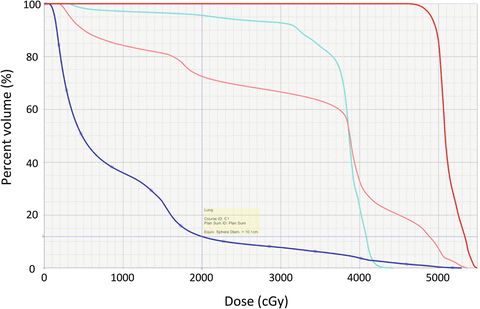

Fig. 13.7
DVH analysis of a four-field technique for a middle thoracic esophagus tumor (50.4 Gy in 28 fraction with elective nodal irradiation of 41.4 Gy). Boost PTV (red), total lung (blue), heart (pink), spinal cord (cyan)
13.2.2.5 Brachytherapy
Brachytherapy permits treatment of a localized area of the esophagus to high radiation doses with relative sparing of surrounding structures. This technique may be used alone or in combination with external beam radiotherapy with or without chemotherapy. The indication of brachytherapy is the treatment of superficial esophageal cancer for curative intent in Japan (local control rate: 79–85 %) [28–34]; on the other hand, it is used to relieve symptom such as dysphagia for palliative intent in the treatment of advanced esophageal cancer in Western countries [35, 36]. Brachytherapy involves intraluminal placement of a radioactive source into the esophagus with an intraorally or intranasally inserted applicator. Brachytherapy can be administered by two general methods: low-dose rate (LDR) brachytherapy and high-dose rate (HDR) brachytherapy. Modern HDR brachytherapy equipment delivers radiation much faster than 12 Gy/h, permitting the delivery of a planned dose within minutes compared with LDR sources, which require many hours or days. As a general rule of HDR brachytherapy, the diameter of the balloon applicator should be 15–20 mm. The whole length of tumor and 2 cm above and below the lesion are included in the target volume. The reference dose point is set at a depth of 5 mm of the esophageal submucosa (5 mm beyond the wall of the balloon surface). There is no definite consensus about the optimal dose of intraluminal brachytherapy for esophageal cancer. In Japan, 50–60 Gy external beam radiotherapy followed by 8–12 Gy in 2–4 fractions (3–4 Gy per fraction) HDR brachytherapy is generally used. It was reported that higher dose per fraction is associated with the risk of esophageal ulcer and perforation [29]. Dose of 4 Gy or less per fraction by HDR brachytherapy and dose of 6 Gy or less per fraction by LDR brachytherapy once or twice a week is recommended in Japan [32]. The American brachytherapy society (ABS) recommends an HDR dose of 10 Gy in two fractions, prescribed at 1 cm from the source, to boost 50 Gy EBRT [37]. Figure 13.8 illustrates the dose distribution and 3D view in the treatment planning of HDR brachytherapy.
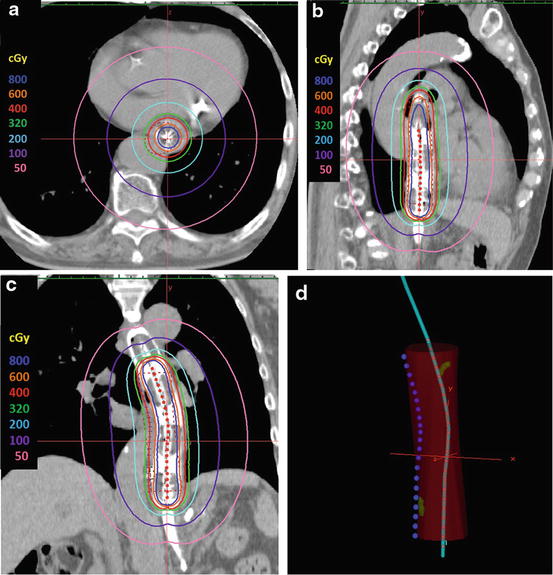

Fig. 13.8
Dose distribution of intraluminal brachytherapy for a cT1bN0 middle thoracic esophagus tumor. Prescription dose: 400 cGy at a depth of 5 mm of the esophageal submucosa as the reference dose point. (a) axial view. (b) sagittal view. (c) coronal view, (d) 3D-view. Clips (green), high-risk CTV (red): GTV plus 2 cm margin proximally and distally along the length of the esophagus, reference dose point (blue), catheter (cyan), dwell points (red)
13.3 Results
13.3.1 Radiotherapy Alone
Radiation therapy alone has been usually delivered when lesions are deemed inoperable because of tumor extent or medical contraindications. In general, patients receiving radiation as a sole treatment modality have a median survival of 6–12 months and 5-year survival of <10 %. In a review of 49 early series involving more than 8,400 patients treated with radiation therapy alone, overall survival rates at 1, 2, and 5 years were 18, 8, and 6 %, respectively [38]. Hancock and Glatstein reviewed 9,511 patients and found that only 5.8 % were alive at 5 years [39]. Okawa and colleagues reported 5-year survival rates by stage [40]. For patients with stage I disease, the 5-year survival rate was 20 %; stage II, 10 %; stage III, 3 %; and stage IV, 0 %. Overall, the 5-year survival rate was 9 %. For cervical esophageal lesions treated with radiation alone, the cure rates are comparable with those in patients treated with surgery alone. As a result of clinical trial, Radiation Therapy Oncology Group (RTOG) trial (RTOG8501) comparing combined chemotherapy with 5-FU and cisplatin with radiotherapy (50 Gy) versus radiotherapy alone (64 Gy) showed that 3-year survival with radiotherapy alone was 0 % [1–3]. In a prospective trial of radiotherapy alone (66 Gy) for patients older than 80 years old with T1-T3N0M0 squamous cell carcinoma of the thoracic esophagus, median survival time and 3-year overall survival rate were 30 months and 39 %, respectively [41].
13.3.2 Chemoradiotherapy
The landmark trial establishing the superiority of concurrent chemoradiotherapy to radiation therapy alone was RTOG8501. Herskovic and colleagues reported the results of this randomized trial comparing combined chemotherapy with 5-FU and cisplatin with radiotherapy (50 Gy) versus radiotherapy alone (64 Gy) for esophageal cancer (88 % SCC) [1]. The median survival in patients treated by radiation alone was 8.9 months compared with 12.5 months for those treated with combined therapy, with 2-year survival rate 10 versus 38 %; the incidence of local recurrence decreased from 24 to 16 %, and the 2-year distant metastasis rate decreased from 26 to 12 %. Updated results showed that at 5 years, survival rates were 26 and 0 %, respectively, for chemoradiotherapy and radiation therapy alone [2, 3].
13.3.2.1 Chemoradiotherapy for Unresectable Locally Advanced Esophageal Cancer
For the locally advanced unresectable esophageal cancer, chemoradiotherapy is the standard treatment with potentially curative intent. Results of clinical trials of definitive chemoradiotherapy for esophageal cancer including T4 are shown in Table 13.2 [6–8, 18, 42–49]. INT0123, a randomized clinical trial, compared standard-dose 50.4 Gy to high-dose 64.8 Gy with both concurrent 5-FU and cisplatin chemotherapy for patients with clinical T1-4N0-1M0 esophageal cancer [18]. This study was closed after interim analysis showed no probability of superiority in the high-dose arm. No significant difference in median survival (18.1 vs. 13 months), 2-year survival (40 vs. 31 %), or local-regional failure/persistence of disease (52 vs. 56 %) was seen between the standard-dose and high-dose arms. Eleven treatment-related deaths occurred in the high-dose arm compared with 2 in the standard-dose arm, with 7 of the 11 high-dose arm deaths occurring in patients who received 50.4 Gy or less. In a single institute phase II trial of chemoradiotherapy with 5-FU and cisplatin and 60 Gy irradiation for patients with clinical T4 and/or M1 lymph node ESCC, complete response (CR) rate was 33 % and median survival time and 3-year survival rate were 9 months and 23 %, respectively [6]. Another clinical trials of 5-FU and cisplatin and 60 Gy irradiation for patients including clinical T4 showed that CR rate was 15–33 % and 2-year, 3 year survival rates were 27–46 % and 23–30 %, respectively [7, 8, 42–44]. Other combination regimen using new drugs (paclitaxel, docetaxel, oxaliplatin, S-1, and cetuximab) with concurrent radiotherapy have been evaluated [46–49].
Table 13.2
Results of clinical trials of definitive CRT for ESCC including T4
Author | cStage | Pathology: rate of SCC (%) | No. of pt. | Regimen | CR rate | Survival |
|---|---|---|---|---|---|---|
INT0123 [18] (USA) | T1-4N0-1 (T4: 8 %) | 86 | 109 | FP + 50.4 Gy | NR | 2 years: 31 % |
109 | FP + 64.8 Gy | NR | 2 years: 40 % | |||
Ohtsu [6] (Japan) | T4/M1Lym (T4: 67 %) | 100 | 54 | FP + 60 Gy | 33 % | 1 years: 41 % |
3 years: 23 % | ||||||
JCOG9516 [7] (Japan) | T4/M1lym (T4: 100 %) | 100 | 60 | FP + 60 Gy | 15 % | 2 years: 31.5 % |
Nishimura [8] (Japan) | T4/M1Lym (T4: 100 %) | 100 | 28 | FP + 60 Gy | 32 % | StageIII;2 years: 27 % |
StageIV;1 years: 23 % | ||||||
JCOG0303 [42] (Japan) | T4/M1lym (T4: 75 %) | 100 | 71 | FP + 60 Gy | 0 %a | 3 years: 30 % |
71 | Low dose FP + 60 Gy | 1.4 %a | 3 years: 26 % | |||
Stage II–IVA (T4: 44 %) | 100 | 46 | FP + 60 Gy | NR | 2 years: 46 %, 5 years: 35 % | |
45 | Low dose FP + 60 Gy | NR | 2 years: 44 %, 5 years: 22 % | |||
Shahl [45] (Germany) | T3-4N0-1 (T4: 17 %) | 100 | 86 | FLEP → EP + 60 Gy | NR | 3 years: 55 % |
86 | FLEP → EP + 40 Gy + S | NR | 3 years: 58 % | |||
PRODIGE5/ACCORD17 [46] (France) | Stage I–IVA (T4: NR) | 86 | 133 | FP + 50 Gy | 43 % | 3 years: 26.9 % |
134 | FOLFOX + 50 Gy | 43 % | 3 years: 19.9 % | |||
SCOPE1 [47] (UK) | Stage I–III (T4: NR) | 73 | 129 | CP + 50 Gy | 2 years: 56.0 % | |
129 | CP + Cetuximab + 50 Gy | 2 years: 41.3 % | ||||
RTOG0436 [48] (USA) | T1N1/T2-4N0-1/M1a (T3-4: 80 %) | 38 | 169 | Cisplatin + PTX + 50.4 Gy | 59 % | 1 year: 65 %, 2 years: 42 % |
159 | Cisplatin + PTX + Cetuximab + 50.4 Gy | 56 % | 1 year: 64 %, 2 years: 44 % | |||
KDOG0501 [49] (Japan) | T4/M1lym (T4: 69 %) | 100 | 42 | DCF + 50.4 Gy, 61.2 Gy | 52.4 % | 1 years: 66 %, 3 years: 44 % |
13.3.2.2 Chemoradiotherapy for Resectable Esophageal Cancer
Definitive chemoradiotherapy is a treatment option in an attempt to preserve the esophagus for resectable esophageal cancer. Results of clinical trials of definitive chemoradiotherapy for resectable esophageal cancer are shown in Table 13.3 [1–5, 50–52]. For stage I esophageal cancer, Japan Clinical Oncology Group (JCOG) 9708, a phase II trial of chemoradiotherapy with 5-FU and cisplatin and 60 Gy irradiation against primary tumor only, was conducted (Fig. 13.1b). CR rate was 87.5 % and 5-year overall survival rate was 75.5 % [4]. Most of residual or recurrent diseases after chemoradiotherapy were curatively resected by endoscopy or surgery. Several reports showed the efficacy of these salvage treatment after definitive chemoradiotherapy [53–56]. For stage II/III esophageal cancer, JCOG9906, a phase II trial of chemoradiotherapy with 5-FU and cisplatin and 60 Gy irradiation with elective lymph nodal irradiation, showed promising activity with 62.2 % of CR rate and 36.8 % of 5-year overall survival rate [5]. Acute toxicities were mild, but there were four treatment-related death (5.3 %) related to late toxicities. Moreover, 8–15 % of high mortality rate was seen in patients who underwent salvage surgery to residual or recurrent disease after chemoradiotherapy [55, 56]. Late toxicity and higher mortality rate might be caused by the extensive radiation field and daily treatment of AP/PA opposite fields. Therefore, a phase II trial of chemoradiotherapy with 5-FU and cisplatin and concurrent radiotherapy 50.4 Gy using of multiple field technique with reducing both the radiation dose and the volume of heart within the radiation field for stage II/III esophageal cancer was conducted [51]. At a median follow-up of 29.4 months, late toxicities which were greater than grade 3 were observed in 5.9 % of pneumonitis only. And CR rate was 70.6 % and 3-year overall survival rate was 63.8 %.
Table 13.3
Results of clinical trials of definitive CRT for resectable (non T4) ESCC
Author | cStage | Pathology: rate of SCC (%) | No. of pt. | Regimen | CR rate | Survival |
|---|---|---|---|---|---|---|
T1-3N0-1 | 88 | 62 | 64 Gy | NR | 2 years: 10 %, 5 years: 0 % | |
134 | FP + 50 Gy
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|