16 Radiation Oncology
Introduction
In general, the treatment for breast cancer consists of surgery with or without radiation and with or without systemic therapy. Most women with breast cancer have two distinct local therapy options: mastectomy or breast-conserving therapy (BCT), which is defined as limited surgery and adjuvant whole breast irradiation. The choice of local therapy depends on several factors, not the least of which is patient preference. In a review of over 16,000 patients with stages I and II breast cancer treated in 1994, Morrow and associates reported that only 42% were treated with BCT.1 Using 11 SEER (Surveillance Epidemiology and End Results) registries of women diagnosed with breast cancer between 1988 and 1999, researchers found that 51% and 49% were treated with mastectomy and breast-conserving surgery (BCS), respectively.2 Ninety-six percent of the patients undergoing BCS also received adjuvant radiation. In contrast, researchers in France reported that in 2002 78% of eligible breast cancer patients were treated with BCT.3 The increase in BCT over the years is perhaps due to the comfort of patients and physicians with the results of several randomized trials comparing BCT with mastectomy. Although some studies failed to show an improvement in overall survival with BCT, it is clear that outcomes in overall survival are similar with mastectomy and BCS. In addition, there have been reports of improved quality of life in women treated with BCT when compared with those treated with mastectomy.4 The indications and contraindications of radiation after BCS and mastectomy are discussed in the following sections.
Traditionally, radiation therapy follows surgery, but the sequencing with systemic therapy may have more variability. The sequencing of radiation and chemotherapy does not appear to affect disease-free or overall survival. The term systemic therapy refers to chemotherapy, hormonal therapy, and biologics (e.g., trastuzumab). These agents may be administered at different times during the management of breast cancer. They may be administered before definitive surgery (neoadjuvant), after surgery but before radiation, concurrent with radiation, or after radiation. The sequence chosen depends on the agent used and its associated toxicities. There is no clear evidence that sequencing affects overall survival.5–7 Neoadjuvant chemotherapy has been most influential in predicting a patient’s ultimate response to chemotherapy and increasing the rates of BCT. In the National Surgical Adjuvant Breast and Bowel Project (NSABP) trial B-18, 27% of the patients originally considered ineligible for BCT and for whom mastectomies were planned were able to have lumpectomies following tumor shrinkage as a response to chemotherapy.6–9
Breast-Conserving Therapy
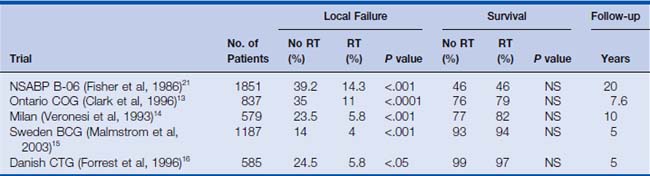
The most common use of therapeutic radiation with respect to breast cancer is in BCT. There have been seven randomized prospective trials in women with invasive breast cancer comparing mastectomy with limited breast surgery (lumpectomy) with or without adjuvant whole breast irradiation.10,11 These trials showed not only that there were similar survival rates between the mastectomy and lumpectomy patients but also that whole breast irradiation after lumpectomy significantly reduced the incidence of local failures when compared with that for patients treated with lumpectomy alone (Table 16-1). Based on the strength of these findings, the National Cancer Institute (NCI) put forth the following consensus statement: “Breast conservation treatment is an appropriate method of primary therapy for the majority of women with stage I–II breast cancer and is preferable because it provides survival rates equivalent to those of total mastectomy and axillary dissection while preserving the breast.”17
Invasive Disease and Breast-Conserving Therapy
Several prospective randomized trials have shown that local failure (LF) after lumpectomy alone may be as high as 30% to 40%. However, if radiation is administered, local failure is reduced to approximately 6% to 15%9,11–14 (see Table 16-1). Perhaps the most quoted American study is the NSABP B-06. In this trial, women with tumors smaller than 4 cm were randomized to mastectomy or lumpectomy with or without adjuvant radiation. With 20 years’ median follow-up, there was no difference in overall survival among the three treatment groups. However, there was a significant difference in local failure between the conservative surgery groups. The incidence of ipsilateral breast recurrence was 14% in the women who underwent lumpectomy and radiation compared with 39% in the women who underwent lumpectomy alone.21
Approximately 75% of all local failures occur within the first 5 years after treatment. However, late local recurrences have been reported at 15 to 20 years after treatment. Factors predictive of local failure after conservative breast surgery and radiation include positive/close margins, young age (less than 35 to 40 years old), extensive intraductal component, and lymph vascular invasion.22–24
Until recently, most evidence supported equivalent overall survival rates in patients treated with either modified radical mastectomy (MRM) or lumpectomy with or without radiation. However, there is now evidence to the contrary. The Early Breast Cancer Trialists’ Cooperative Group (EBCTCG) evaluated seven prospective randomized trials comparing BCS with and without adjuvant radiation. With 15 years’ follow-up, the authors showed a statistically significant 5% absolute survival benefit in women who received radiation after BCS compared with survival in women who underwent BCS alone.10 Another meta-analysis, by Vinh-Hung and Vershraegen, showed a similar statistically significant survival benefit of 8.5% in favor of adjuvant radiation therapy.25 It is now clear that radiation not only reduces local recurrence after BCS but also provides a real survival benefit when compared with BCS alone.
Ductal Carcinoma in Situ and Breast-Conserving Therapy
Unlike invasive breast cancer, there are no randomized trials comparing BCT with mastectomy in patients with ductal carcinoma in situ (DCIS). The closest study to an ideal randomized trial was a trial essentially by error. When reviewing the pathology of patients treated in NSABP B-06, a trial in which patients were randomized to lumpectomy alone, lumpectomy and radiation, or mastectomy, it was discovered that some of the patients enrolled in this invasive breast cancer trial in reality had DCIS.12 The DCIS patients were evenly distributed among the three treatment groups. With a median follow-up of 39 months, the local failure rates in the lumpectomy patients with and without adjuvant radiation were 7% and 23%, respectively. There was no difference in survival rates among the three groups. The lack of survival differences suggests that DCIS could be equally well managed with mastectomy or conservative surgery and radiation.
Additional support for BCT in patients with DCIS comes from two large randomized prospective trials comparing lumpectomy alone with lumpectomy and radiation—NSABP B-17 and European Organisation for Research and Treatment of Cancer (EORTC) 10853 (Bijker 2006).26,27 Both trials revealed that the rate of local recurrence was significantly reduced by approximately 50% with adjuvant radiation. In B-17, with a median follow-up of 8 years, the rates of local recurrence in the lumpectomy alone and lumpectomy and radiation groups were 31% and 13%, respectively. In the EORTC trial, with a median follow-up of 10 years, the local failure rates were 15% and 26% in patients who did and did not receive adjuvant radiation, respectively. Factors associated with an increased rate of local failure in patients with DCIS receiving BCT include young age, solid and cribriform subtypes, positive margins, and symptomatic detection of DCIS.28–32
Alternative Breast-Conserving Therapies
Partial Breast Irradiation
Because of the physical and social costs of whole breast irradiation, there has been much interest in developing ways of facilitating BCT.33–39 One such method is partial breast irradiation (PBI). Because most local recurrences after BCT have been in the vicinity of the original tumor, one may question the need to treat the whole breast. Several large ongoing randomized trials in both North America and Europe are attempting to answer this question. In general, there are three ways to deliver radiation to the lumpectomy bed plus the margin while minimizing the dose to the remainder of the breast (PBI). The methods can be divided into two general techniques: brachytherapy and teletherapy. Brachytherapy is the placement of radioactive source close to, next to, or through the target organ/tissue. Teletherapy refers to treatment in which the radioactive source is at some distance from the target organ/tissue. The benefit of brachytherapy is that the radiation dose decreases rapidly with increasing distance from the source. Thus, this technique is uniquely suitable for treating small areas with high doses while sparing surrounding tissue.
Brachytherapy may be delivered via interstitial or intracavitary techniques.37,40,41 The interstitial technique consists of placing hollow needles through the breast and lumpectomy bed in multiple planes. At some later time, radioactive sources are placed in those needles to treat the lumpectomy bed. This can be achieved with low-dose-rate or high-dose-rate brachytherapy. In the intracavitary brachytherapy technique, a balloon is placed in the lumpectomy bed at the time of surgery. The balloon is inflated to approximate the walls of the cavity. A source is then placed in the balloon to deliver radiation to the walls of the lumpectomy bed. High-dose-rate brachytherapy is commonly used in the intracavitary technique.
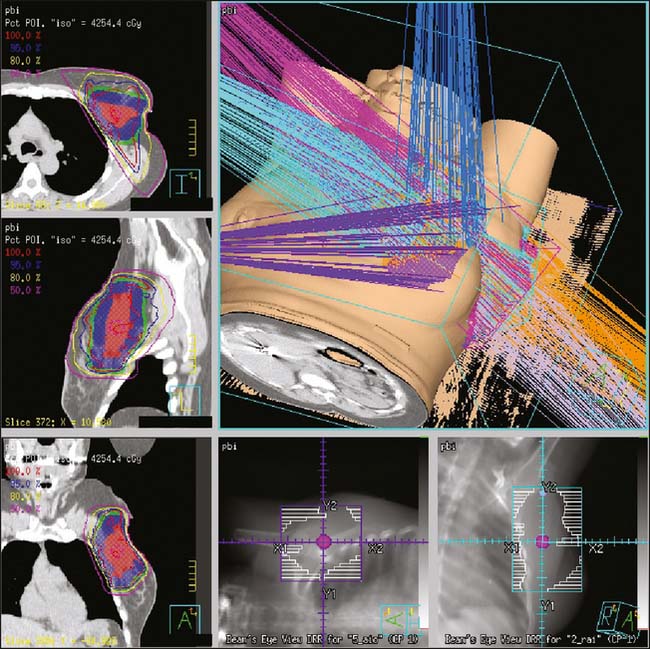
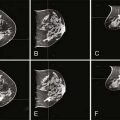
The remaining PBI techniques are via an external source (teletherapy). Teletherapy PBI is delivered either intraoperatively or postoperatively. The intraoperative technique delivers one large dose of radiation to the exposed lumpectomy bed with either photons or electrons.42–44 The postoperative technique uses threedimensional conformal or intensity-modulated radiation therapy (IMRT) to deliver radiation to the lumpectomy bed with margin.38 The duration of postoperative PBI may range from 5 days of twice-daily treatments to 15 once-daily fractions.38,45,46 See Figure 16-1 for an example of postoperative external beam PBI.
All of the latter PBI techniques concentrate on decreasing the duration of the radiation portion of BCT. However, if one considers the entire adjuvant course of therapy for many women, then decreasing only the radiation is of little consequence to those facing several months of systemic therapy. In the hope of facilitating the entire course of adjuvant breast cancer therapy, researchers at Johns Hopkins are combining PBI with concurrent dose-dense chemotherapy (doxorubicin and cyclophosphamide) (PBICC).45 A typical course of chemotherapy every 3 weeks followed by adjuvant radiation can range from 5 to 7 months or longer. With PBICC, the complete course of chemotherapy and radiation could be reduced to just 2 to 4 months. Although PBI and PBICC appear attractive, one must remember that they are still experimental therapeutic techniques and regimens. Trials rigorously comparing PBI with standard whole breast irradiation are in progress. Until long-term follow-up is available, PBI and PBICC should not be offered off-study.
Lumpectomy Alone for Invasive Breast Cancer
Several studies attempted to identify a subset of breast cancer patients for which radiation is not necessary. Despite these many attempts, all studies showed that radiation reduced the rate of local failure when compared with limited surgery alone. However, recent studies suggest that certain elderly women may safely consider foregoing radiation. In a trial by Hughes and colleagues, women older than 70 years were randomized to receive adjuvant radiation or no radiation after lumpectomy for estrogen receptor-positive tumors less than 2 cm.35 Nodal status was not evaluated in all patients. All patients received tamoxifen. With 5 years of follow-up, the authors reported a statistically significant difference in local failure of 1% in favor of radiation compared with 4% with no radiation. The authors argue that the absolute local failure difference between the two arms is small; thus, radiation could be safely avoided in this group.35 Fyles and coworkers reported a similarly designed trial that evaluated the efficacy of adjuvant radiation in women older than 50 years. In this trial, with a median of 5.6 years, the rate of local failure was 0.6% and 7.7% in those who did and did not receive adjuvant radiation, respectively.36 In both studies, there was a several-fold increase in local control with the addition of radiation. Although this is promising, longer follow-up is needed, and currently conservative surgery alone may be best considered for the elderly woman with multiple comorbidities whose life expectancy is relatively short.
Lumpectomy Alone for Ductal Carcinoma in Situ
Once factors are identified as predictive of local failure after BCT, it is only natural to attempt to identify patients who have a risk of local failure so small that they may avoid radiation. Probably the most well-known decision tool in this regard is the Van Nuys Prognostic Index (VNPI) first published by Silverstein and colleagues.47 This prognostic index is used to divide patients into three risk groups, each with different needs for radiation or mastectomy. Unfortunately, though very attractive, the VNPI has not been reliably verified in other studies. Mascarel and associates were unable to reproduce the VNPI results, nor were Fisher and coworkers when this classification system was applied to patients treated in NSABP B-17.29,48 Nonetheless, there is such great interest in defining a group that could be treated with conservative surgery alone that there are two large American trials addressing this topic. Eastern Cooperative Oncology Group registry trial E5194 monitored patients with DCIS who refused radiation after lumpectomy.49 The other trial sponsored by the Radiation Therapy Oncology Group (RTOG) 98-04, randomizes women with DCIS to radiation or no radiation, with or without adjuvant tamoxifen.
Although the results of RTOG 98-04 are not yet available, early results of E5194 have been published.50 Eligible patients (n = 711) who were entered in this study had to have a low-/intermediate-grade DCIS of 2.5 cm or less or a high-grade DCIS of 1.0 cm or less. All patients had to have a 3.0 mm or greater margin. After a median 5.4 years of follow-up, rates of 6.1% and 14.8% of ipsilateral breast events were found in patients with low-/intermediate- and high-grade DCIS, respectively. The authors state that carefully selected patients with low-/intermediate-grade DCIS treated with surgery alone have an acceptably low rate of ipsilateral breast events. Patients with high-grade DCIS do not appear to be candidates for conservative surgery alone. However, they also recognize that these results are preliminary and that further follow-up is necessary.
Contraindications to Breast-Conserving Therapy (Invasive or DCIS)
Although the NCI consensus statement declares that the majority of women with early-stage breast cancer are eligible for BCT, one must ask who are the minority of women for which BCT is not an option? In other words, what are the contraindications to the use of BCT in this group? These contraindications may be divided into three general categories: unacceptable cosmetic result, unacceptable complication rate, and high probability of recurrence (Box 16-1). We briefly discuss here each of these categories.
Box 16-1 Relative Contraindications to Breast Conservation Therapy
The main motivation behind BCT, as the name implies, is preservation of the breast while maximizing survival. However, if the preserved breast is cosmetically unacceptable, the patient has not fully benefited from this therapy. Therefore, factors associated with poor cosmetic outcome are considered contraindications to BCT. An unacceptable cosmetic result may occur in women with small breasts who undergo complete resection of a large tumor. In these patients, there is very little remaining breast tissue, and they would be better served with a mastectomy and reconstruction. Women with very large breasts are also at risk of having a poor cosmetic outcome, since it is technically difficult to deliver a homogeneous dose of radiation. As a consequence of the large differential doses across the breast, these women may develop fibrosis and other soft tissue toxicities associated with radiation. Surgical treatment factors associated with poor cosmetic outcome include large volume of resection, subareola resection, and poor scar orientation. Radiation treatment factors that may affect cosmetic outcome include fraction size, total dose, type of radiation, and concurrent chemotherapy and radiation therapy.51–57 For these reasons, one must use optimal surgical and radiation techniques.
Another category of contraindications to BCT addresses factors associated with a high rate of complications. These complications may also affect cosmetic outcome. Previous radiation to the breast is considered a contraindication because it has been associated with fibrosis and tissue necrosis.58 However, evidence suggests that, in certain cases, additional limited radiation may be possible.59,60 Another factor associated with an unacceptably high rate of complications is the presence of collagen vascular diseases (connective tissue diseases) such as systemic lupus erythematosus or scleroderma.61–63 BCT, in the setting of connective tissue disorders, may also result in breast fibrosis or tissue necrosis. Note that the literature supporting a link between collagen vascular disease and increased radiation toxicity is somewhat tenuous. The conclusions are not uniform, and the published reports contain relatively small numbers of patients.61,62 Thus, some refer to collagen vascular diseases as a relative contraindication. Finally, because of the fear of complications, pregnancy is also included in this category. Although studies have shown that the theoretical exposure to the fetus during breast/chest wall irradiation would be minimal, many still consider pregnancy an absolute contraindication to radiation therapy.64,65 A pregnant woman may have a lumpectomy or mastectomy and receive chemotherapy after the first trimester.66 After delivery, she may then receive breast irradiation. With the advent of neoadjuvant chemotherapy, one could argue to treat the pregnant patient with chemotherapy until she delivers and then to perform either a lumpectomy or mastectomy.
The third and final category of contraindications specifically addresses in-breast recurrence risk. When the risk of a recurrence in the breast is greater than 30% to 40% at 5 years, perhaps the patient is not well served by BCT and may be better served by mastectomy. This is especially true when one considers the growing literature linking in-breast recurrence with increased mortality.67 Factors associated with a high rate of in-breast recurrence after BCS and radiation include positive or close margins, multiple sites of disease involving more than one quadrant (multicentric disease), lymph-vascular invasion (LVI), and invasive disease with an extensive DCIS component.11,31,68,69 Patients who have the BRCA1 or BRCA2 mutation do not have a higher rate of in-breast failures and thus are candidates for BCT.70,71 Women younger than 35 to 40 years have an increased rate of local failure when compared with that of their older counterparts for reasons that are not exactly clear.30–32 Nonetheless, when BCT is compared with mastectomy, in younger patients there is no difference in overall survival.72 Therefore, young age alone is not a contraindication to BCT.
Breast-Conserving Technique
In most of the studies previously discussed, BCS was followed by whole breast irradiation, which did not routinely include regional nodes (supraclavicular, axillary, and internal mammary lymph nodes). Some of the lower axillary nodes are included in the breast tangents by default. However, there are occasions in which regional nodal irradiation is purposely added to whole breast radiation therapy. At our institution, the indications for nodal radiation are presently extrapolated from the postmastectomy radiation therapy trials and are discussed in the following section.
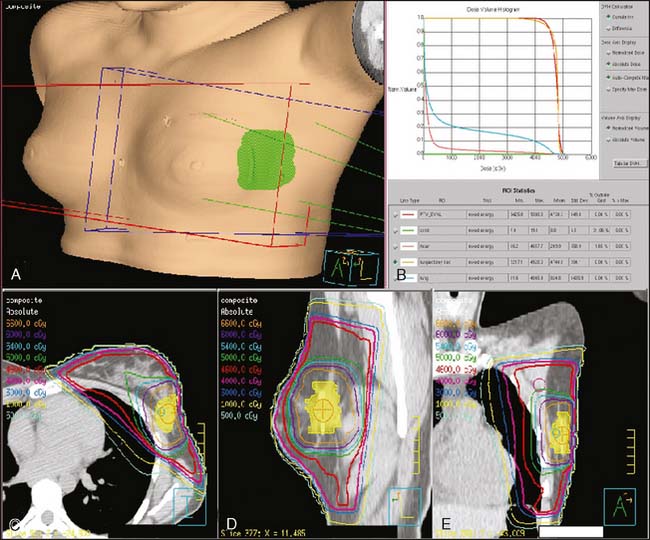
Typically, the entire breast is treated to a microscopic disease dose of 45–50 Gray in 25–28 fractions. The tumor bed then receives an additional 10–16Gy (boost). Two prospective randomized trials have shown an improvement in local control when patients received a boost to the lumpectomy bed after whole breast radiation.73,74
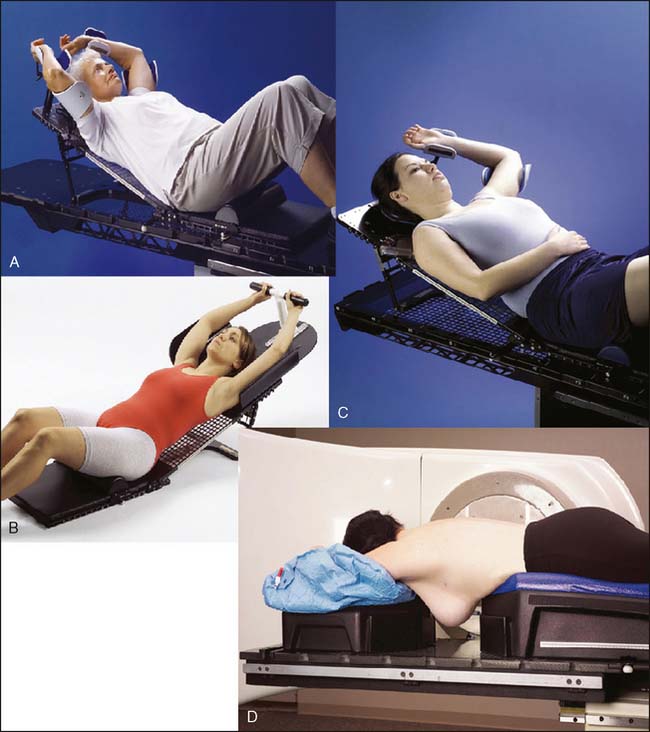
See Figures 16-2 and 16-3 for examples of patient positioning and planning for standard breast-conserving therapy.
Postmastectomy Radiation
The second most common use of therapeutic radiation in the management of breast cancer occurs after mastectomy (postmastectomy radiation [PMXRT]). Some of the earliest randomized prospective trials in oncology addressed the role of radiation after mastectomy for breast cancer. These early trials clearly showed a local control benefit with postmastectomy radiation. Unfortunately, evidence of a survival benefit was lacking. In fact, a meta-analysis of these early trials showed a survival detriment.75 Most of these deaths were believed to be associated with radiation damage to the heart. As a consequence, PMXRT was reserved only for the most advanced cases.
In 1997 and 1999, three randomized prospective trials of PMXRT were published. All three trials showed not only a local control benefit with PMXRT, as already seen in many of the earlier trials, but also a statistically significant survival benefit. These trials, in contrast to their predecessors, benefited from modern standardized radiation therapy techniques as well as from modern chemotherapy (Table 16-2).18–20,76
The high rate of local failure is at the core of the remaining criticisms. In all three trials, an unusually high rate of local regional failure was seen in the patients with one to three positive lymph nodes who did not receive radiation—30% to 33%. This rate stands in contrast to a local regional failure rate of 13%, as reported in a review of many prospective American trials.77 Some have argued that inadequate surgery, specifically an inadequate axillary dissection, may be the cause of the high rate of local failure in these trials. As a consequence, radiation compensated for less than ideal surgery. An attempt to specifically address these concerns was made via a large phase III trial randomizing women with one to three positive lymph nodes to radiation or no radiation. Unfortunately, because of poor accrual, the trial was closed prematurely.
The controversy over whether to offer PMXRT to patients with one to three positive lymph nodes may soon be ending (Table 16-3).18–20,78,79 In 2006, the Early Breast Cancer Trialists’ Cooperative Group presented the results of a meta-analysis of more than 3000 women with pathologically proven one to three positive lymph nodes, treated with mastectomy and axillary clearance with or without adjuvant radiation. The majority of the patients received systemic therapy. In this study, the authors reported a statistically significant overall survival benefit in favor of adjuvant radiation.80 This survival benefit was also seen in women with four or more positive nodes. The report strongly suggests that when deciding whether to offer adjuvant radiation therapy, the distinction made among one and three and four or more positive lymph nodes should be abandoned. Consequently, we offer PMXRT to all women with any number of positive axillary lymph nodes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree