As recognition of the importance of QOL as a patient-centered outcome has increased over time (see Fig. 14.2), the measurement of QOL as a patient-reported outcome has evolved. Numerous measures that ask patients to rate their response to questions about specific domains of QOL currently exist. However, the value of the information provided by measures of QOL is contingent upon the quality of the measure. Good measures of QOL should be validated, have good reliability, be sensitive to change, and be accepted by clinicians and patients. In an effort to regulate claims of drug effectiveness in improving QOL, the Food and Drug Administration (FDA) has further addressed concerns about QOL measurement [6]. According to the FDA Oncologic Drug Advisory Committee Quality of Life Subcommittee, pharmaceutical company claims of improved QOL must be specific to the QOL domain that was measured, with the recommendation that assessment of specific symptoms is an appropriate way to improve measurement of QOL domains [7].
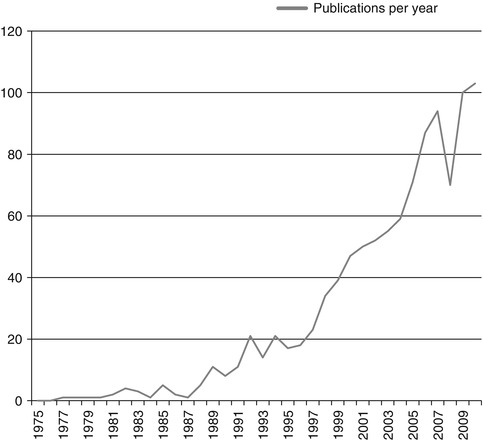

Fig. 14.2
Number of references with “quality of life” and “ovarian cancer” in Pubmed search from 1975–2010
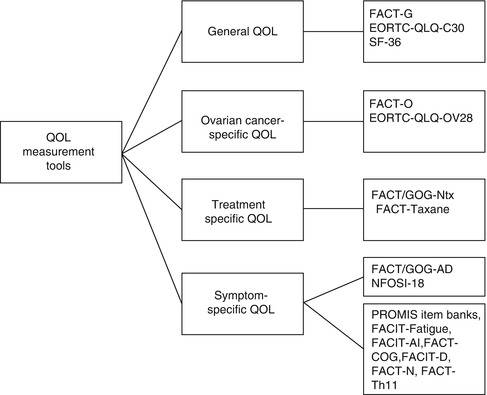
The validated instruments most commonly used to assess QOL in ovarian cancer include both general measures of QOL as well as ovarian cancer-specific measures of QOL. Additionally, a number of symptom-specific measures of QOL have recently been developed for use in women with ovarian cancer and provide a focused assessment of disease- and treatment-related symptoms. Figure 14.3 displays the categories of QOL measures and measures most commonly used in ovarian cancer.
General QOL Measures
Several psychometrically sound general measures of QOL have been used to assess QOL in ovarian cancer. The Functional Assessment of Cancer Therapy-General (FACT-G [1]) is a 27-item general measure of QOL in cancer that assesses four domains of well-being: physical well-being, functional well-being, emotional well-being, and social well-being. The FACT-G can be used alone or in combination with disease and/or treatment-specific subscales. The European Organization for the Research and Treatment of Cancer QLQ-C30 (EORTC QLQ-C30 [8]) is a 30-item general measure of QOL in cancer that is comprised of five functional subscales, three symptom subscales and a global health and QOL scale. Like the FACT-G, it can be used alone or combined with cancer-specific modules. Finally, the Short-Form 36 health survey (SF-36 [9]) is a generic measure of QOL that has been widely used in numerous disease and treatment conditions. It consists of 36 items comprising eight subscales which form two higher-order scales: the Physical Component Summary (PCS) and the Mental Component Summary (MCS).
Ovarian Cancer-Specific QOL Measures
Several instruments have been developed to measure aspects of QOL specific to women with ovarian cancer. The Functional Assessment of Cancer Therapy-Ovarian (FACT-O [10]) consists of the FACT-G plus an ovarian cancer-specific subscale. The 11-item ovarian subscale was developed with input from ovarian cancer patients and gynecologic oncology clinical experts. The FACT-O provides a reliable and valid assessment of QOL in ovarian cancer that is sensitive to change. The Physical Well-Being scale, Functional Well-Being scale, and the Ovarian Cancer Subscale can be combined to create the Trial Outcome Index (TOI), which is frequently used in clinical trials to assess change in physical/functional outcomes. In addition to the FACT-O, the European Organization for the Research and Treatment of Cancer Quality of Life Ovarian Cancer (QLQ-OV28 [11]) is a 28-item ovarian cancer symptom-specific questionnaire module developed to supplement the EORTC QLQ-C30. The EORTC QLQ-OV28 measures abdominal/gastrointestinal symptoms, neurotoxicity, chemotherapy side effects, hormonal symptoms, body image, perception of disease/treatment, and sexual functioning and demonstrates good reliability and validity [11].
Treatment-Specific QOL Measures
A number of valid and reliable instruments have been developed to assess the QOL issues associated with specific types of treatment. The 11-item Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Neurotoxicity (FACT/GOG-Ntx [12, 13]) questionnaire measures peripheral neuropathy secondary to platinum/paclitaxel chemotherapy. Similarly, the Functional Assessment of Cancer Therapy-Taxane (FACT-Taxane [14]) is a 16-item questionnaire that measures the symptoms and QOL effects associated with the taxane class of chemotherapeutic agents. Although these measures were not developed for specific use among women with ovarian cancer, given that platinum/paclitaxel is a common chemotherapy regimen for ovarian cancer, they provide an opportunity to assess treatment impact on QOL in ovarian cancer.
Ovarian Cancer Symptom-Specific QOL Measures
In response to the FDA’s statement that pharmaceutical company claims of improved QOL must be specific to the QOL domain measured, several valid and reliable instruments have recently been developed to measure ovarian cancer-specific symptoms and their impact on QOL. The Functional Assessment of Cancer Therapy/Gynecologic Oncology Group-Abdominal Discomfort (FACT/GOG-AD [15]) is a four-item measure of abdominal discomfort that was created in an effort to more accurately measure patients’ perceptions of abdominal discomfort and its impact on QOL. The FACT/GOG-AD was created in response to evidence suggesting greater pain among intraperitoneal chemotherapy recipients [16]. It can be used to measure differences in treatment response as well as symptoms and complications resulting from both disease and treatment in ovarian cancer.
A recent addition to the measures of ovarian cancer-specific symptoms and QOL is the National Comprehensive Cancer Network-Functional Assessment of Cancer Therapy Ovarian Symptom Index-18 (NFOSI-18 [17]). The NFOSI-18 is an 18-item symptom index that reflects the symptoms rated as highest priority by both oncology clinical experts and women with advanced ovarian cancer (see Table 14.1). It was developed to address the potential discrepancy between symptoms reported as high priority by oncologists and patients in an effort to provide a more comprehensive perspective on symptoms and QOL in advanced ovarian cancer. It is comprised of three subscales: disease-related symptoms, treatment-related symptoms, and general function/well-being. Preliminary evidence suggests good reliability and validity [17].
Table 14.1
Rankings of top five symptoms/concernsa by advanced ovarian cancer patients with comparison to oncology clinician symptom ratings
Symptom/concern | Patient overall rank | % Patients endorsed top 5 | Oncology clinician overall rank |
|---|---|---|---|
Lack of energy (fatigue) | 1 | 61 | 1 |
Able to sleep well | 2 | 37 | – |
Able to enjoy life | 3 | 25 | – |
Content with current QOL | 4 | 24 | 7 |
Able to get around by myself | 5 | 22 | – |
Constipation | 5 | 22 | – |
Nausea | 5 | 22 | 4 |
Pain | 8 | 20 | 3 |
Worry that condition will get worse | 8 | 20 | 5 |
Feeling ill | 10 | 18 | – |
In addition to these ovarian cancer-specific symptom indices, several non-disease-specific instruments measure symptoms of relevance among women with ovarian cancer. Table 14.2 lists instruments from the Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT) Measurement System which can be administered individually or in combination with the FACT-O. Additionally, the National Institutes of Health (NIH) Patient-Reported Outcomes Measurement Information System (PROMIS) initiative has resulted in the development of a host of item banks measuring QOL-related domains in chronic illness, such as physical function [18], pain [19, 20], sleep [21], emotional distress [22], and social health [23]. The availability of psychometrically sound measures, such as those described, enables a better understanding of the impact of symptoms on QOL in ovarian cancer.
Table 14.2
Non-disease-specific symptom measures from the Functional Assessment of Chronic Illness Therapy (FACIT) Measurement System
Symptom domain | Measure | Number of items |
|---|---|---|
Fatigue | Functional Assessment of Chronic Illness Therapy-Fatigue Scale (FACIT-Fatigue [87]) | 13 |
Ascites | Functional Assessment of Chronic Illness Therapy-Ascites Scale (FACIT-AI [88]) | 13 |
Diarrhea | Functional Assessment of Chronic Illness Therapy-Diarrhea Scale (FACIT-D [88]) | 11 |
Cognitive function | Functional Assessment of Cancer Therapy-Cognitive Function Issues Scale (FACT-Cog [88]) | 13 |
Neutropenia | Functional Assessment of Cancer Therapy-Neutropenia Scale (FACT-N [89]) | 19 |
Thrombocytopenia | Functional Assessment of Cancer Therapy-Thrombocytopenia (FACT-Th11 [36]) | 11 |
Anemia | Functional Assessment of Cancer Therapy-Anemia/Fatigue (FACT-An [90]) | 20 |
Symptoms and QOL in Ovarian Cancer
Women with ovarian cancer are at risk for diminished QOL due to the disease itself, as well as aggressive treatments such as abdominal surgeries and chemotherapy [2, 24]. While these therapies may have antineoplastic benefits, they can also result in a host of toxicities, which, along with disease-related symptoms, have the potential to adversely affect a variety of QOL domains. Table 14.3 lists common symptoms affecting QOL in ovarian cancer.
Table 14.3
Ovarian cancer symptoms affecting quality of life
Symptom category | Symptoms |
|---|---|
Physical | Fatigue |
Neurotoxicity | |
Neutropenia | |
Abdominal pain/discomfort | |
Alopecia | |
Nausea/vomiting | |
Thrombocytopenia | |
Anemia | |
Cognitive changes | |
Sexual and reproductive | Decreased interest in sexual activity |
Decreased sexual pleasure | |
Increased sexual discomfort | |
Increased fertility concerns | |
Psychological | Depression |
Anxiety | |
Anger | |
Changes in social functioning | |
Impaired sleep quality |
Physical Symptoms
QOL is adversely affected by ovarian cancer symptoms; in fact they are a part of the QOL concept itself in the context of ovarian cancer. Disease, treatment, and psychological factors can result in elevated rates of fatigue among women with ovarian cancer [25], which is the most consistently endorsed high-priority symptom by women with advanced disease [17]. Fatigue has been defined as a persistent, distressing, subjective sense of tiredness related to cancer treatment that is not in proportion to recent activity and which interferes with typical functioning [26]. Fatigue may continue to present difficulties even well after the completion of treatment, as indicated by the finding that 32.7 % of ovarian cancer survivors at least 6 months posttreatment continued to report difficulty due to fatigue [27]. This finding highlights the importance of ongoing monitoring of fatigue even among women no longer actively receiving treatment for ovarian cancer.
Neurotoxicity represents another common physical symptom that negatively impacts QOL in women with ovarian cancer. Neurotoxicity is a symptom often associated with cisplatin or taxane chemotherapy regimens. Neurotoxicity symptoms associated with these chemotherapy regimens include burning, numbness, tingling, and shooting sensations in the extremities [28] and may interfere with functional abilities and activities of daily living. Neurotoxicity may also persist beyond the completion of chemotherapy. A recent randomized controlled trial revealed that one-third of women receiving cisplatin and paclitaxel had persistent neuropathy 12 months after starting treatment [29], and neurotoxicity was significantly greater at 12 months among women who received intraperitoneal chemotherapy compared to those who only received intravenous chemotherapy. At the present time, there is a paucity of therapies that have been effective in preventing or treating neurotoxicity [30, 31], although novel taxanes may offer promise of reduced neurotoxicity, compared to paclitaxel [32].
Abdominal discomfort and pain may also adversely impact QOL in women with ovarian cancer. Abdominal symptoms are common in ovarian cancer and may be related to disease-related factors, such as bloating and ascites [5], or treatments such as surgery or intraperitoneal chemotherapy [33]. A recent randomized controlled trial found that patients receiving only intravenous chemotherapy had significantly more improvement in abdominal discomfort from baseline to cycle four when compared to patients receiving chemotherapy via both intravenous and intraperitoneal administration [15]. However, it should also be noted that no differences in abdominal discomfort existed between intravenous-only and intravenous/intraperitoneal patients by 1 year follow-up [34].
Myelosuppression, including anemia, neutropenia, and thrombocytopenia, represents another physical symptom that may negatively affect QOL in ovarian cancer. Myelosuppression is a common toxicity associated with the platinum-based chemotherapies, as well as paclitaxel. Anemia may also be related to disease-related factors and is associated with numerous symptoms impacting QOL, including fatigue, dizziness, dyspnea, activity intolerance, headaches, concentration difficulties, and sleep disturbance [35]. Severe anemia may require treatment with erythropoietin or red blood cell transfusion, which may delay treatment and confer additional threat to QOL. Neutropenia related to cancer enhances susceptibility to infection and may result in fever and hospitalization. Neutropenia is a common dose-limiting toxicity of taxane [5], and may also result in delay of chemotherapy administration. Thrombocytopenia is associated with concerns such as bruising, bleeding, fatigue, and worry, which may, in turn, affect activity level [36]. Thrombocytopenia is standardly treated with platelet transfusions and may be associated with delays in treatment and dose reductions.
Impaired cognitive functioning can also have an adverse effect upon QOL. Although research has begun to explore changes in cognitive functioning in ovarian cancer, it is not well understood and is likely due to multiple etiologies. Proposed mechanisms of cognitive impairment in individuals receiving chemotherapy include chemotherapy-induced neurotoxicity associated with demyelination, secondary inflammatory response, and microvascular injury [37]; chemotherapy-induced disruption of hippocampal neurogenesis [38] is associated with decreased cerebral gray and white matter volume; [39] anemia, fatigue, and neuroimmunologic changes [40]; and comorbid psychiatric or neurologic conditions [41]. Recent research has begun to explore cognitive functioning in women undergoing treatment for ovarian cancer using objective, cognitive, and neuropsychological assessments (See Table 14.4). Several of these studies found no evidence of a decline in cognitive functioning [42, 43]; one noted a significant improvement in short-term attention from baseline to posttreatment [42], and several found that scores on cognitive tests ranged from average to high average when compared with published normative values [43, 44]. This is in contrast to the findings of Hess and colleagues, who reported a decline in cognitive functioning over time in the majority of participants [41]. The reason for the variability in these findings remains unclear; however, it is possible that the use of different measures or domains of cognitive functioning may account for these contrasting findings. Questions remain about the discrepancy between patient-reported cognitive impairment and results from neuropsychological assessments suggesting little impairment.
Table 14.4
Studies that have assessed cognitive functioning in women with ovarian cancer
Author | Design | N | Participants | Cognitive domains assessed | Findings |
|---|---|---|---|---|---|
Correa et al. [44] | Cross-sectional | 48 | Long-term ovarian cancer survivors who were either in remission or with recurrent disease | Attention | There were no significant differences between the two groups in any cognitive domain. Although group mean cognitive scores were in the average range, 28 % of participants met criteria for cognitive impairment |
Executive function | |||||
Learning | |||||
Memory | |||||
Hess et al. [41] | Prospective | 27 | Women with ovarian cancer receiving platinum-based chemotherapy | Attention | 92 % of participants demonstrated cognitive impairment from baseline to cycle 3. 86 % demonstrated cognitive impairments from baseline to cycle 6 |
Processing speed | |||||
Reaction time | |||||
Hensley et al. [43] | Prospective | 27 | Women with ovarian cancer receiving combination therapy with paclitaxel, carboplatin, and gemcitabine | Attention | Scores on cognitive function tests ranged from average to high average on all tests across time, with no decline in cognitive function |
Concentration | |||||
Cognitive flexibility | |||||
Psychomotor speed | |||||
Mayerhofer et al. [42] | Prospective | 28 | Women with ovarian cancer receiving combination paclitaxel/carboplatin | Motor skills | Scores on a test assessing short-term attention significantly improved from pre- to post-chemotherapy. There were no other significant changes over time in scores from the other cognitive tests administered |
Short-term attention | |||||
Numerical memory |
Sexual and Reproductive Symptoms
Disease- and treatment-related factors are commonly associated with sexual and reproductive symptoms that impair QOL in women with ovarian cancer. Specifically, nerve damage from surgery, removal of tissues important for sexual activity, surgical menopause, and estrogen deficiency may negatively impact sexual activity and functioning in women with ovarian cancer [5]. Findings suggest that approximately half of ovarian cancer survivors are sexually active [45, 46]. Among ovarian cancer survivors who were not sexually active, a lack of interest/desire in sex and physical problems were cited significantly more frequently as reasons for sexual inactivity, when compared to a normative sample [45, 46]. Moreover, sexually active ovarian cancer survivors reported significantly greater levels of sexual discomfort and significantly lower levels of sexual pleasure compared to a normative sample [45]. These findings are noteworthy, given recent evidence from Von Gruenigen and colleagues that the majority of participants with ovarian cancer indicated significantly impaired interest and satisfaction with sexual activity [47].
Survivors of ovarian germ cell tumors may experience a slightly different profile of sexual symptoms than survivors of epithelial ovarian cancer. Ovarian germ cell tumors are typically diagnosed in younger women of childbearing age and are associated with more favorable treatment outcomes and cure rates [48]. Table 14.5 displays the results from a recent comparison of sexual and reproductive functioning among survivors of ovarian germ cell tumors and matched controls. Although survivors of ovarian germ cell tumors did not differ from matched controls in menstrual/gynecologic difficulties or sexual discomfort, they reported significantly less sexual pleasure [48]. In addition, the fertility impact of cancer may be especially salient in survivors of ovarian germ cell tumors. Gershenson and colleagues found that the majority of survivors of ovarian germ cell tumors who received fertility-sparing surgery followed by combination chemotherapy reported experiencing regular menstrual cycles [48]. However, compared to age-, race-, and education-matched controls, survivors of ovarian germ cell tumors had significantly more reproductive concerns, and, as expected, infertile survivors of germ cell tumors had significantly greater reproductive concerns than fertile survivors [48].
Table 14.5
Comparison of reproductive and sexual functioning in survivors of ovarian germ cell tumors and matched controls
Ovarian germ cell tumor survivors (N = 132) | Matched controls (N = 137) | ||||
|---|---|---|---|---|---|
Reproductive/sexual domain | Mean | SD | Mean | SD | p |
Menstrual/gynecological symptoms | 18.2 | 6.8 | 17.0 | 4.6 | .123 |
Reproductive concernsa | 15.7 | 12.1 | 8.5 | 7.1 | <.0001 |
Sexual pleasureb | 9.9 | 5.5 | 11.8 | 4.3 | .017 |
Sexual discomfortb | 1.3 | 1.7 | 1.0 | 1.3 | .154 |
Sexual self schemac | 55.8 | 13.4 | 55.8 | 11.8 | .983 |
Psychological Symptoms
The psychological sequelae of ovarian cancer, which is described in greater detail in a separate chapter of this book, may also pose a threat to QOL. For example, in one study, 21 % of a sample of women with ovarian cancer met clinical cutoff for depression, and 29 % scored above the 75th percentile for anxiety [49]. Indeed, there is some evidence to suggest that anxiety poses a greater problem than depression among women with ovarian cancer [47, 50]. For example, women with advanced ovarian cancer rated “worry that condition would worsen” among their highest-priority symptoms [17], and in one sample of women with ovarian cancer, 55 % reported a fear of dying [51]. It is possible that treatment-related factors may impact anxiety symptoms in ovarian cancer. For example, a greater proportion of women who underwent suboptimal debulking reported more severe anxious symptoms compared to women who underwent optimal debulking [47].
Implications of QOL Assessment
Given the impact of symptoms on QOL, QOL is an important end point when assessing the effects of cancer treatment. Measuring QOL provides the ability to examine how well treatment ameliorates disease-related symptoms, as well as whether treatment-related toxicities outweigh the potential clinical benefits. The assessment of QOL may also serve as a prognostic factor across the disease trajectory. For example, two independent randomized controlled trials have found QOL to be predictive of survival outcomes in ovarian cancer. In a randomized controlled trial of interval secondary cytoreduction, Wenzel and colleagues found that QOL at the third chemotherapy cycle predicted overall survival in women with advanced ovarian cancer [29]. In a randomized trial comparing cisplatin/cyclophosphamide and cisplatin/paclitaxel chemotherapy, Carey and colleagues found that better baseline global QOL predicted longer progression-free survival and overall survival, independent of performance status [52]. These findings suggest that perhaps QOL may also serve as an indicator of which patients may not respond well to treatment. Another clinical implication of assessing QOL is the opportunity for enhanced patient-provider communication surrounding treatment decision-making and symptom management. As previously noted, patients and oncology clinicians may differ in terms of the symptoms and aspects of QOL they perceive as most important [17]. Thus, assessing and discussing QOL enables oncology providers to develop a better understanding of the patients’ perception of QOL as it pertains to their treatment. Incorporating QOL into discussions regarding treatment decision-making is especially important in situations where different treatments demonstrate similar survival benefits but different toxicity profiles.
In addition to the benefits of QOL assessment in the clinical setting, there are also benefits of incorporating QOL measurement into ovarian cancer research. This is reinforced by the National Cancer Institute (NCI) and the FDA, both of which have recently asserted that the improvement of survival and QOL should be the aims of cancer research [53]. Given that approximately three-fourths of women with ovarian cancer are diagnosed with advanced disease [54], as well as the limited curative treatment options for advanced disease, the improvement and maintenance of QOL is an important outcome in clinical trials. Clinical trials that include QOL as an outcome can measure treatment success by the extent to which the treatment manages or improves ovarian cancer-specific symptoms affecting QOL.
QOL and Recent Advances in the Treatment of Ovarian Cancer
The preservation and improvement of QOL represents an important aim of clinical trials investigating advances in treatment modalities for ovarian cancer. The development of validated QOL measurement tools has enabled clinical trials to collect and report on QOL data in the context of newly emerging treatment modalities. In recent years, a number of treatment advances have emerged, including new chemotherapeutic agents, innovations in the timing and mode of chemotherapy administration, and new surgical procedures. Research has increasingly sought to identify how these new treatment strategies affect QOL in women with ovarian cancer, and efforts are necessary to continue to more comprehensively incorporate measures of QOL in trials assessing novel therapies. Table 14.6 summarizes recent research evaluating new treatment modalities that has included QOL as an end point.
Table 14.6
Measures of QOL used in recent clinical trials of treatment for ovarian cancer
Study | QOL measure | QOL outcomes |
|---|---|---|
Greimel et al. [58] | EORTC QLQ-C30 | Paclitaxel/carboplatin arm experienced better QOL than paclitaxel/cisplatin arm |
Pokrzywinski et al. [55] | FACT-O | Sequential treatment arm experienced better QOL than combination treatment arm |
Wenzel et al. [34] | FACT-O | Intraperitoneal arm experienced worse QOL during and shortly after treatment, compared to the intravenous-only arm. No significant differences in QOL between groups at 1 year |
FACT/GOG-Ntx | ||
FACT/GOG-AD | ||
Vergote et al. [64] | EORTC QLQ-C30 | No significant difference in QOL between primary debulking and neoadjuvant chemotherapy arms |
EORTC QLQ-Ov28 | ||
Wenzel et al. [29] | FACT-O | No significant difference in QOL between participants who underwent interval secondary cytoreduction and those that did not |
Maintenance Chemotherapy
Despite improvements in treatment, the majority of women with ovarian cancer will recur within 5 years of treatment [55], and there is evidence to suggest that most women will relapse within 18 months of completion of first-line treatment [32]. Despite the availability of chemotherapeutic agents for relapsed disease, response rates diminish with each relapse due to drug resistance over time [32]. Maintenance chemotherapy has been proposed as a potential solution; however, the potential for improved survival with maintenance chemotherapy must be considered in light of additional toxicity from prolonged treatment and its potential impact on QOL. It has been proposed that symptoms associated with maintenance therapy such as fatigue, myalgias, myelosuppression, alopecia, and neuropathy may impact QOL [32]. Although maintenance therapy using novel taxanes may confer benefit with less impact on QOL, no studies to date have reported on QOL in maintenance therapy in ovarian cancer, and though toxicity data has been used as a proxy measure of QOL, it does not fully capture patients’ perceptions of QOL [32]. An ongoing phase 3 trial examining monthly taxane maintenance therapy includes QOL as a secondary end point [56], and future research on the QOL outcomes in maintenance chemotherapy trials is needed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree