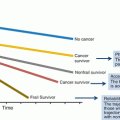
Fig. 17.1
Conceptual framework for receipt of optimal cancer care (Adapted from Shavers and Brown, JNCI 2002 with permission)
After the initial treatment decisions are made, and treatments are underway, many women want to know what is coming next, and that is where formal survivorship care planning for the post-treatment period has emerged as a major gap in quality care (Hewitt et al. 2006; Ganz and Hahn 2008; Ganz et al. 2008). For almost a decade since the Institute of Medicine (IOM) report on adult cancer survivorship care (Hewitt et al. 2006), various organizations have worked diligently to improve the post-treatment communication and coordination of care, specifically at the transition between active treatment and follow-up care. In breast cancer patients, this usually occurs at the end of adjuvant chemotherapy and/or radiation therapy. For many women, extended adjuvant endocrine therapy will be prescribed; this is a critical and lifesaving component of treatment and not all women understand the importance of this therapy. Non-adherence to oral endocrine therapy is very common and results in poorer breast cancer outcomes (Hershman et al. 2010, 2011). Non-adherence can occur because of uncontrolled and bothersome symptoms, as well as concerns about the financial cost of treatment. Failure to adhere to this treatment is an important quality of care issue, and lack of communication and trust in physicians doing the follow-up care can contribute to this (Kahn et al. 2007).
Breast cancer survivorship does not occur in a vacuum. As discussed elsewhere in this volume, although the average age of breast cancer incidence is 61 years, about 25 % of incident cases are in women younger than 50 years and the majority are over 65 years of age. Life stage, partnership status, financial and other resources, including the generosity of health insurance plans, may contribute substantially to the quality of the survivor’s life after cancer. In addition, continuing symptoms (e.g., menopause related, sexuality and intimacy concerns, fatigue, and depression) can disrupt relationships and the ability to work and care for children. The human cost of breast cancer is substantial. Finally, as described in Chap. 16 in this volume on living with metastatic breast cancer, there are more than a 100,000 women living for extended periods of time on cancer directed therapy for whom active disease and its consequences (e.g., pain, physical limitations, treatment toxicities) further complicate the quality of life. We hope these introductory remarks set the stage for a more detailed discussion of the challenges of delivering high quality care to breast cancer patients and survivors.
Challenges for Survivors: Dealing with the Costs of Cancer Care
Covering the costs of cancer care is a major concern for many cancer survivors. Cancer-related medical costs have accelerated at a rate beyond those of other medical treatments (Vanchieri 2005). It is projected that US health care spending will reach $4.3 trillion and account for 19 % of the national gross domestic product by 2019 (Schnipper et al. 2012). This increase has been driven by a dramatic rise in both the cost of therapy and the extent of care, especially in the last few months of life. Physicians directly or indirectly control or influence the majority of cancer care costs, including the use and choice of drugs, the types of supportive care, the frequency of imaging and the number and the extent of hospitalizations (Smith and Hillner 2011). In addition there are numerous unmeasured costs associated with loss of work and subsequent loss of insurance. Given the long life expectancy of patients with breast cancer, it is not surprising that total costs of breast cancer care in both the metastatic and non-metastatic setting can be higher than other cancers.
Patients are most directly affected by out-of-pocket costs, which have increased as more therapies have switched from intravenous to oral therapies. It is estimated that more than one quarter of the 400 antineoplastic agents now in the pipeline are oral drugs. Oral cancer therapies are often advertised as being more convenient than parenteral therapies as they can reduce patient travel, eliminate time spent in the infusion center, and avoid issues related to intravenous access. However there are a number of concerns about oral therapies that have arisen. As with other new cancer therapies, they are accompanied by increased costs and financial burdens for patients (Vanchieri 2005; Benson et al. 1998). Total prescription medication costs exceeded $234 billion by 2008, an annual rate of increase of over 10 % (Kaiser Family Foundation 2010). Some of the most expensive oral cancer drugs are used to treat patients with breast cancer, such as everolimus, which can cost $100,000 or more per year. The financial burden borne by patients prescribed these drugs can be very high, with co-pays running from hundreds up to thousands of dollars per month. It is known that as out of pocket costs increase, the likelihood of compliance with medications decreases, which can adversely affect survival outcomes. It is well-known that adherence to hormonal therapy is a large problem in breast cancer, and co-payment amount has an independent effect on adherence and early discontinuation of hormone therapy (Neugut et al. 2011). Furthermore, the cost of oral supportive care medications, such as anti-emetic therapies, can be prohibitive for some patients, resulting in unnecessary toxicity and decreased quality of life. Not surprisingly, out-of-pocket expenses are the largest in countries of low and lower-middle income, despite the fact that people in these countries have the lowest resources to cover these extra costs (Anderson et al. 2011).
A major challenge as the number of cancer survivors increases will be to provide cost effective follow-up care by reducing overuse of unnecessary tests and procedures so that access to effective medications can be preserved. Public health efforts, such as the Cancer Treatment Fairness Act, which requires insurance to cover oral cancer treatment medications the same as they cover intravenously and injected cancer treatment medications, may increase drug price transparency, improve access and reduce out of pocket costs for life-saving cancer treatments. Efforts at decreasing economic disparities in breast cancer care are especially important given the rapid increase of expensive oral cancer therapies.
Disparities in Breast Cancer Treatment and Outcome
Patient, provider, and systems barriers contribute to delays in cancer care, lower quality of care, and poorer outcomes in vulnerable populations, including low income, underinsured, and racial/ethnic minority populations (Fig. 17.2). Compared to non-Hispanic white women, overall breast cancer incidence is lower among black women but breast cancer mortality is higher (about 40 %) with trends that vary depending on age and location (American Cancer Society 2011). The racial difference in outcome has increased over time, and may reflect disparities in diagnosis and treatment. Despite the fact that nationally the use of mammography is nearly equivalent for blacks and whites (American Cancer Society 2013), black women have a much higher rate of incidence before the age of 40 years, are more likely to be diagnosed with larger tumors (>5.0 cm), and have higher rates of distant-stage disease at diagnosis (American Cancer Society 2011). Differences between blacks and whites also exist with regard to access to clinical trials and innovative cancer treatments (Sateren et al. 2002; Tejeda et al. 1996); receipt of biomarker testing, follow-up care post-treatment, and surveillance mammography (Shavers and Brown 2002).
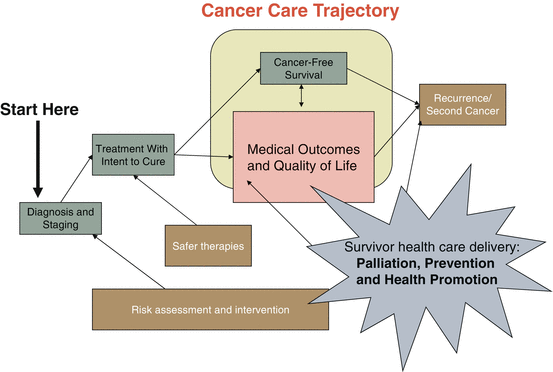

Fig. 17.2
Model depicting the role cancer survivorship care and the cancer care trajectory
The factors contributing to the striking difference in mortality between blacks and whites (Li et al. 2003; Wheeler et al. 2013) are likely to be multi-factorial and complex, and have been attributed to differences in tumor biology (Bowen et al. 2006; O’Brien et al. 2010; Carey et al. 2006; Lund et al. 2009), psychological, behavioral factors, and social factors and access to care (Magai et al. 2008; Gerend and Pai 2008; O’Brien et al. 2010; Du et al. 2007, 2008), and access to and response to new adjuvant treatments including hormonal therapy (Menashe et al. 2009; Jatoi et al. 2003; Caudle et al. 2010). Interestingly, when you look at differences in survival between races over time, the separation began in the 1980s and has continued since that time, as white women have had improvements in breast cancer survival and black women have not. This separation coincides with an increased understanding of the importance of adjuvant treatment, and suggests that this disparity is modifiable (2010). Also of interest, recent studies suggest that the evolution of racial disparities in breast cancer survival are different in different cities in the US, which may be a reflection of state level screening programs, access and public health education (Hunt et al. 2014).
Recent studies have suggested that black women more often did not receive timely treatment compared to other women (Shavers and Brown 2002); are less likely to receive optimal systemic adjuvant therapy than white women (Hassett and Griggs 2009; Bickell et al. 2006); and are more likely to have delays in the initiation of adjuvant chemotherapy and radiotherapy, which are all associated with worse survival (Hershman et al. 2006a, b). Despite the fact that black women are more likely to have triple negative breast cancer, the racial disparities gap is greatest among the hormone-sensitive subtypes of breast cancer and because non-adherence to anti-estrogen treatment has been shown to adversely impact survival, differences in the utilization of this treatment may well explain some of the black-white breast cancer mortality disparity (Shavers and Brown 2002).
In low and middle-income countries advanced stages at presentation and poor diagnostic and treatment access contribute to lower breast cancer survival than in higher income countries (Harford et al. 2011). In 2010 the Breast Health Global Initiative reported an executive summary of their consensus meeting. Challenges for improving outcomes include little community awareness that breast cancer is treatable, inadequate pathology services for diagnostics, fragmented treatment options and establishment of data registries to show progress with interventions (Anderson et al. 2011).
Much work has been done to define the problem and establish modifiable factors that may contribute. Efforts going forward will need to focus on interventions and public policy changes to reduce the disparity in outcome by intervening in factors that can easily be modified.
Research Underway to Address These Issues and Future Strategies/Policy
Reduce Overdiagnosis and Over Treatment
With the expansion of population based mammography screening described earlier, there has been a stage shift to earlier stage breast cancer, as well as an explosion in the detection of non-invasive ductal cancers or ductal carcinoma in situ (DCIS). In 2013, there were expected to be 64,640 DCIS cases and 232,340 cases of invasive cancer (DeSantis et al. 2014). The continued increase in diagnosis of DCIS has not reduced the number of invasive cases of breast cancer and is felt to be overdiagnosis of precancerous disease that would not likely become invasive or not be detected and cause death (Esserman et al. 2009, 2013; Esserman and Thompson 2010). This also leads to over treatment, as patients with DCIS are subjected to the same local therapy approaches (i.e., surgery and radiation therapy) that are applied to patients with invasive cancer. Moreover, the psychological distress associated with the diagnosis of DCIS is substantial (Ganz 2010).
Reduction in the age of initiation of screening mammography to the 40–50 years age group, as well as the interval frequency for mammography screening in women over 50 years remains controversial, in spite of evidence based reviews that suggest that starting at age 50 years is sufficient, and that the interval can be less frequent than annually. Among the biggest challenges with DCIS is the identification of high risk disease that would in fact lead to significant morbidity and mortality, or the converse, those women who need minimal if any treatment. In the case of stage I hormone receptor positive breast cancer, where low risk disease patients can avoid chemotherapy, there have been only a few trials that have looked at the omission of radiation therapy, and the uptake of avoiding this therapy has been limited (Giordano 2012). The NRG clinical trials group is hoping to do a large simple trial to address this question in early stage patients. This will reduce both morbidity and cost if treatment can be avoided. Future trials will hopefully be able to incorporate genomic and molecular markers to identify high and low risk patients and to tailor the intensity of treatment to the tumor characteristics.
Among the many challenges we face in this area is changing the beliefs of women regarding the value of mammographic screening, given the 30 years campaign by various health professional organizations supporting the use of this technique for early detection of breast cancer (Welch and Passow 2014). Women and their physicians are reluctant to give up the idea that earlier detection of a cancer will make a difference. Translating data from the population to the individual patient is challenging, and patients do not want to be denied a procedure that they think might be lifesaving. The same applies to other imaging technologies that may be used for screening, staging or monitoring of breast cancer, such as breast MRI and PET-CT scans. These currently have no role in the management of breast cancer, with exception of breast MRI in women at high risk for breast cancer (e.g., BRCA1/2 gene carriers), but their use is widespread, and in fact, the use of breast MRI may be contributing to the recent increase in bilateral mastectomy, even when unilateral breast conserving treatment would be appropriate. Whether these choices are rational, or driven by overzealous treatment recommendations of physicians, is uncertain. The ability to perform immediate breast reconstruction at the time of initial treatment, and the advances in cosmetic results (e.g., nipple sparing surgery), have encouraged both patients and physicians to opt for this therapy. The misunderstanding and confusion about the appropriateness of this extreme therapy for high risk gene carriers (e.g., Angelina Jolie) and not for the general population of women with breast cancer has increased the demand for this treatment. Unfortunately, many physicians go along with these approaches, and it is uncertain whether these recommendations are independent of financial considerations. Finally, in the post-treatment phase, many women cannot accept the fact that surveillance testing for recurrence is unproven (Khatcheressian et al. 2013), and it is easier for physicians to offer testing, than to spend time discussing the lack of value in these assessments. Everyone feels better when the blood work and scans come back normal, but when the tumor marker or imaging provides a false positive result, much anxiety and additional testing results. ASCO and other professional societies are participating in the ABIM Foundation Choosing Wisely campaign, which have identified these types of services as being of low value and a target for quality improvement efforts.
Survivorship Care Plans: Assessment and Referral to Appropriate Clinicians
One of the recently promoted means of improving the coordination of care for breast cancer survivors has been the use of survivorship care planning (Ganz and Hahn 2008), and a care plan document, as a means of summarizing what treatments have been received, what surveillance is needed to identify recurrence, and how to manage persistent symptoms that do not resolve in the post-treatment period, as well as be on the lookout for rare but important late effects of treatment. With the IOM report on adult cancer survivors in 2005 (Hewitt and Ganz 2006; Hewitt et al. 2006), there was a flurry of activity to try to move forward with the idea of care plans. Sadly, it has had relatively modest uptake in clinical practice, but the most widely studied cancer has been breast cancer (Tevaarwerk et al. 2014; Birken et al. 2014; Haq et al. 2013). It was not until the recent decision by the American College of Surgeons Commission on Cancer to set survivorship care planning as a standard for accreditation in 2015 that clinical cancer delivery settings have identified strategies to make this happen. One of the authors has been extensively involved in the dissemination of care plans during the past decade, and it is good that we are finally seeing some uptake. Nevertheless, the care plan document, which has been the focus of many studies, is not really the issue. It is the communication and coordination of care that is critically important as part of the post-treatment care planning. With the anticipated work shortages for all oncology health professionals and the increasing number of cancer cases expected in the next decade (Institute of Medicine 2013), medical and surgical oncologists will have limited space in their practices to provide ongoing care for early stage, low risk breast cancer patients, and they must develop strategies to share the care with primary care providers who can continue the monitoring of these patients while addressing age-related comorbid conditions as well as persistent cancer treatment related symptoms such as fatigue, menopausal symptoms, depression and others. As called for in the recent IOM report on the delivery of high quality cancer care (Institute of Medicine 2013), coordination of care will be absolutely essential to deliver high-quality cancer care. Breast cancer patients are an ideal target for innovations in the delivery of quality care.
In addition to post-treatment care planning and coordination of care, we need to emphasize the importance of psychosocial care services and palliative care in breast cancer survivors. This is extensively called out in the recent IOM report on quality of care (Institute of Medicine 2013) as being an essential component of high quality cancer care from the time of diagnosis. Among the breast cancer survivors that present for consultation at UCLA, almost all have had superb and technically appropriate medical care. However, few if any have had their psychosocial needs addressed and many are seeking help for severe and debilitating symptoms (e.g., fatigue, cognitive dysfunction, neuropathy) that are not being addressed in the routine follow-up care they are receiving, usually by at least 2–3 oncology specialists. Some research (see Chaps. 5, 6, 7, 8, 9 on various symptoms), is beginning to identify genetic or behavioral risk profiles that may allow us to identify patients at high risk for persistent post-treatment symptoms. Possible strategies in the future may include a battery of questionnaires and blood tests that will facilitate risk profiling and allow tailoring of treatments and/or early interventions to reduce the risk of persistent problems. Much more prospective observational, biopsychosocial data collection will be necessary. Ideally this should be conducted within the setting of clinical trials, but might be feasible within the learning health care systems of the future (Abernethy et al. 2010; Institute of Medicine 2013).
To accomplish these goals, we must build and develop a knowledge base as well as improve the self-efficacy among primary care providers who have not been heavily engaged in the follow-up care of cancer patients for several decades (Cheung et al. 2013; Han et al. 2013; Potosky et al. 2011; Blanch-Hartigan et al. 2014). Primary care providers would like to share the care of cancer patients with oncologists and find the idea of care plans very attractive and helpful to them (Shalom et al. 2011; Hewitt et al. 2007). Here again, breast cancer is an excellent model for this type of shared care. There are many women’s health primary care providers who have large numbers of breast cancer survivors in their practices. These physicians often have an interest in menopause-related issues, such as vasomotor symptom management and bone health. This makes them excellent primary care providers for the follow-up of the post-treatment, low risk breast cancer patient. With specific recommendations about which cancer surveillance tests are necessary, and when the oncologist needs to re-engage in care, these providers can manage breast cancer survivors very competently, as has been shown in several randomized trials (Grunfeld et al. 1996, 2006). Oncology professional societies must lead the way in working with other health care provider professional groups to develop curricula and educational strategies to enhance the competencies of all care providers who will be following breast cancer survivors in the post-treatment period (2013).
Improving Quality and Reducing Health Care Disparities
In recent years there has been progress in increasing screening rates for breast cancer, however, despite this, the gap between white and black breast cancer mortality rates is still widening because early detection does not reduce mortality unless those diagnosed are subsequently treated in a timely and effective way. One interventional approach has been through patient navigation. Patient navigation refers to the individualized assistance offered to patients, families and caregivers to help overcome healthcare systems barriers and facilitate timely access to quality medical care (Freeman and Wasfie 1989). Patient navigation has repeatedly been shown to improve rates and timeliness of follow-up of cancer screening abnormalities in various populations (Paskett et al. 2011). Less is known about treatment adherence, satisfaction with care and survival. The challenge will be to figure out the best implementation among patients with the greatest need in a cost-efficient manner. Other interventions are currently being tested to improve adherence to hormone therapy for breast cancer such text messaging and email reminders.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree