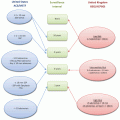
Start small but start immediately
Identify a process to improve
Plan
Define performance metrics and collect baseline data
Do
Develop and implement an action plan
Study
Review the results of the intervention
Act
If the intervention is ineffective, repeat the cycle with another intervention
If the intervention proves effective, go to the next step
Repeat the cycle
Use this process to address the next problem
This chapter discusses the recommended, standardized techniques for performing and reporting CTC that have been derived from peer-reviewed, evidence-based research. In addition, this chapter introduces the development of CTC quality metrics and a data registry for instituting a cyclical quality improvement program into clinical practice.
Brief History of CTC
Early validation trials of CTC in enriched patient cohorts demonstrated sensitivity of 90 % detection of 1 cm and greater polyps and 98 % detection of colorectal cancer [8, 9]. Initial clinical uses of CTC began as an adjunct diagnostic examination following incomplete optical colonoscopy, largely in patients with redundant colons or obstructive cancers. Since 2007, CTC has had Medicare coverage in 47 states for diagnostic indications, predominantly in patients following incomplete colonoscopy or in patients at risk for colonoscopy [10]. This coverage decision, also supported by private insurance, was largely based on the diagnostic accuracy of the exam to detect colorectal polyps and cancer, along with its low-risk profile.
By early 2000, validation trials in asymptomatic cohorts began to show evidence that CTC could be used for colorectal screening. In 2003, the multicenter Navy trial of 1233 asymptomatic patients reported results of per-polyp sensitivities for detection of ≥ 6 and ≥ 10 mm polyps of 89 and 94 %, respectively [11]. In 2008, the multicenter trial of American College of Radiology Imaging Network (ACRIN) published similar results in a more diverse trial of 15 centers, including academic and private sectors [1]. In this trial, the per-polyp sensitivities for detection of ≥ 6 and ≥ 10 mm polyps were 78 and 90 %, respectively. In 2008, the American Cancer Society jointly with the US Multisociety Task Force on Colorectal Cancer and the American College of Radiology (ACR) endorsed CTC to be used for colorectal screening [12]. In that same time period, the US Preventative Task Force gave CTC an “I” rating (indeterminate), not able to include the ACRIN trial in their meta-analysis, largely due to stated risks of radiation dose, burden of extra-colonic findings, and small polyps left behind in the Medicare population [13].
ACR Practice Parameters for Use of CTC in Adults
After the initial publication of ACR practice guidelines for use of CTC in 2005, the document was revised in 2009, with the most recent update completed in 2014 [3]. These standards define aspects of indications and contraindications of CTC, qualifications of interpreting physicians, specifications of exam techniques, and documentation and reporting of findings.
Indications and Contraindications
The indications for CTC examination in screening individuals include patients who are at average for developing colorectal neoplasia. Considering age-related risk, patients at average risk include patients who are 50 years or older with no symptoms and with no other risk factors. Also managed as low risk are those who have a first-degree relative with colorectal cancer after the age of 60 or multiple second-degree relatives at any age with colorectal neoplasia [12]. Patients with moderate risk include patients who have a first-degree relative with colorectal neoplasia before age 60 or multiple first-degree relatives with colorectal cancer. Moderate-risk patients can be considered for CTC screening in patients who are asymptomatic, in the appropriate clinical context. CTC is not indicated for screening in patients at high risk, which includes patients with inflammatory bowel disease or patients with defined genetic syndromes. It is encouraged that well-defined algorithms for CTC screening indications are defined with community standards among radiologists, gastroenterologists, and referring physicians. As implemented by the large screening program of the Colon Health Initiative at Bethesda, study coordinators or trained radiology or gastroenterology scheduling staff are instrumental in screening patients for current symptoms and family history to ensure that each patient gets triaged into the appropriate screening modality.
The indications for CTC examination for diagnostic indications in symptomatic patients include those who have undergone an incomplete optical colonoscopy in multiple clinical settings, such as abdominal pain, weight loss, gastrointestinal bleeding, anemia, or weight loss. Also for patients who have undergone an incomplete optical colonoscopy, CTC can be done for surveillance of lesions or for further characterization of lesions found to be indeterminate at optical colonoscopy. In addition, diagnostic indications include patients who are at increased risk to undergo optical colonoscopy, such as advanced age, anticoagulant therapy, sedation risk, or prior history of incomplete colonoscopy.
Relative contraindications for CTC and those patients not indicated for CTC are shown in Table 5.2. It is important to perform proper screening of patients for these contraindications before the exam. For same-day incomplete colonoscopy, clear communication between the gastroenterologist and the radiologist is important to convey if deep biopsies or polypectomies are done. If therapeutic interventions have been performed, patients should wait several weeks before undergoing the completion of CTC examination.
Table 5.2
Contraindications for CTC
Relative contraindications for CTC |
|---|
Suspected bowel perforation or high-grade bowel obstruction |
Recent or current colitis or diverticulitis |
Recent colorectal surgery |
Recent deep endoscopic biopsy or polypectomy |
Known colon-containing abdominal wall hernia |
Not indicated for CTC |
Patients at high risk (known genetic syndromes) |
Patients with inflammatory bowel disease |
Evaluation of anal canal disease |
Qualifications of Interpreting Physicians
Proper training of physicians for the performance of CTC is essential for ensuring both the quality and safety of the examination. In 2005, the ACR first issued practice guidelines for the performance of CTC in adults [3]. These practice guidelines provided for the first time a framework of suggested training needed by physicians in order to be able to perform and interpret CTC. Suggested general qualifications for the radiologic technologist to perform CTC are also included.
Fundamental to the ACR standards is that the physician performing CTC assumes responsibility for all parts of the examination. This includes initially ensuring that the study is being performed for an appropriate indication and then making certain that the correct low-dose multidetector CT protocol is employed in a safe manner. The use and volume of oral contrast for tagging of residual material or of intravenous contrast for a diagnostic CTC examination must be monitored. Image reconstructions including multiplanar reformations and three-dimensional (3D) images must be of diagnostic quality for optimal detection of lesions. Finally, accurate interpretation of all images should be recorded in an official report.
Suggested physician training is divided into two broad categories based on previous formal training and ability. The first category is meant for physicians who have prior qualifications in general and/or abdominal–pelvic CT interpretation, such as that attained in an accredited residency and/or fellowship program. This category encompasses standards for those physicians who already meet the qualifications set forth in the ACR practice parameters for performing and interpreting diagnostic CT which helps ensure consistency of qualifications across a broader category within the organization [14]. As a baseline, physicians in this category should have training in radiation biology, the physics of CT scanning, CT image acquisition, and post processing. Prior to specific CTC training, these physicians should already have significant experience in interpretation of CT studies, including the ability to detect extra-colonic findings on CTC studies. If these preliminary qualifications are met, then additional CTC-specific training is recommended, including education in bowel cleansing and insufflation techniques as well as CT image acquisition. Formal interactive training using a computer workstation with dedicated CTC software is required with interpretation, reporting, and/or supervised review of at least 50 colonoscopy proven cases. Hands-on interactive training is critical for developing problem-solving skills using both 2D and 3D images for review of a variety of cases.
The second category is designated for physicians who do not have prior qualifications in general and/or abdominal–pelvic CT interpretation. Physicians in this category need to undergo more intensive educational efforts for learning both about current CT scanning and then specifically about CTC in order to be qualified to safely perform this test within quality standards. This is particularly important since CT scanning uses ionizing radiation and physicians must follow the as low as reasonably achievable (ALARA) principle. In addition to completing an accredited specialty training program, physicians in this category must also document 200 h of continuing medical education in the performance and interpretation of abdominal–pelvic CT and review of 500 CT cases. Similar to physicians in the first category, additional training is needed for CTC including instruction in colonic cleansing, distention, and CTC data acquisition. Formal interactive training of at least 75 colonoscopy proven cases is suggested to include a variety of polyps and cancers.
The maintenance of skills following the initial training period is recommended with review of 50 CTC cases every 2 years. Various methods to accomplish this include actual on-site performance of cases and correlation with follow-up colonoscopy or surgery as well as through attendance at review courses or using electronic media. Maintaining and improving CTC skills may also occur through mentored supervision, double reads, and individual study.
Radiologic technologists are an important part of the CTC team and should be familiar with the correct technical parameters for performing CTC. They should be able to select appropriate CT scanning parameters and help position the patient properly in opposing positions. Technologists should also be able to assist with safe placement and removal of the rectal tube and insufflation of the colon using manual or automated techniques. An important function of the technologist is ensuring a diagnostic study before the patient is released or to alert the physician that additional maneuvers may be needed in order to clear a particular colonic segment.
Specifications of the Exam Techniques
Optimization of CTC technique includes employment of state-of-the-art strategies for each component of the examination. A diagnostic quality of CTC examination includes a properly cleansed and distended colon, allowing the reader to maximize lesion detection ability and to decrease false positives and unnecessary follow-up colonoscopies. Concurrently, multidetector CT parameters should be employed that allow data acquisition, providing excellent image quality balanced with an appropriately low radiation dose. The ACR practice guideline for the performance of CTC in adults includes recommendations for colon preparation and specifics of the examination technique. In particular, suggestions are provided for a quality control program.
Colonic cleansing is required for CTC and consists of ingestion of a saline cathartic and/or polyethylene glycol in combination with dietary limitation on the day before the procedure. Tagging of residual material is suggested using ingested water-soluble contrast alone or in combination with low volume barium. The ingested contrast will increase the density of residual colonic contents allowing easier differentiation from the soft tissue density of polyps and carcinoma. Although a fully cleansed colon is preferred, there is adequate data to support the use of limited cathartic CTC in combination with tagging in the minority of patients who may not be able to comply with or tolerate full catharsis [15–17].
Colonic distention is achieved by the insufflation of room air or carbon dioxide. The preferred method of colonic insufflation is with electronic administration of carbon dioxide which provides reliable and more comfortable colonic distention [18]. If a distended rectal balloon is employed to aid in gaseous retention, careful balloon insufflation is needed. Persistent and severe pain experienced by the patient during rectal balloon insufflation may indicate increased possibility of perforation . Using the scout image of the gas-filled colon in each of two opposing position, it should be ensured that there is complete anatomic scanning of the entire colon. If there is suboptimal distention or collapse of the same colonic segment on supine and prone views, then a limited rescan of the particular segment only may be performed in a decubitus position to assure diagnostic ability of the CTC examination.
Screening CTC consists of a low radiation dose CT of the abdomen and pelvis without the administration of intravenous contrast [19, 20]. A multidetector CT (MDCT) scanner is required to rapidly image patients using a slice thickness of 1–1.25 mm with a breathhold less than 25 s. Heightened awareness of radiation dose from imaging tests over the past several years has helped to ensure that CT scans are performed using the ALARA principle. All CT scanners are required to be able to provide a volumetric CT dose index (CTDIvol) in milligray representing the average radiation dose imparted in the scanned volume. For CTC, this includes dose information from at least two scans, typically the supine scan and the prone scan. The suggested CTDIvol for screening CTC is up to 6.25 mGy per position or up to 12.5 mGy for dual position CTC. This represents one half of the suggested dose limits for routine CT abdomen and pelvis in adults, which is 25 mGy.
Dose reduction strategies are widely available that may be easily applied to CTC examinations. These strategies are particularly important to recognize and implement for screening CTC which is suggested to be repeated at 5-year intervals. Reduction of tube current (milliampere), exposure time (second), tube current–time product (milliampere-second), or tube potential (kilovolt) will result in a decrease in effective radiation dose [21–23]. Other techniques that can significantly reduce radiation dose include automatic dose modulation, image-based noise reduction algorithms and iterative reconstruction techniques [24, 25]. The use of these techniques allows for reduction of CTC effective dose to 3 mSv or less which is equivalent to the average annual background radiation dose and more than 60 % lower than prior screening CTC doses [26].
A quality control program for review of CTC examinations is necessary to identify site-specific areas requiring adjustment or improvement. A minimum colonic cleansing and distention should be adequate for the identification of 10 mm or larger polyps. The radiologic technologist and physician should make certain that the CTC examination is of sufficient quality for diagnosis before the patient leaves so that if an additional limited series is required, it can be performed during the same visit. After starting a CTC program, it is recommended that correlation of radiologic, colonoscopic, and pathologic findings be performed whenever possible. Periodic monitoring of the detection rates of polyps and colorectal cancers should be performed and there should be an evaluation of false-positive rates for reported polyps in patients undergoing follow-up colonoscopy. In conjunction with establishing a quality control program, sites performing CTC are suggested to participate in the ACR National Radiology Data Registry (NRDR) for CTC.
Documentation and Communication of Results
Documentation of CTC results should clearly communicate the presence of clinically significant polyps or cancers found. The size threshold for reporting polyps at CTC is 6 mm, with the surveillance interval for follow-up of a negative or benign CTC examination of 5 years. This is in accordance with the American Cancer Society’s joint colorectal screening guidelines in 2008, which states that polyps 6 mm and greater should be reported at CTC, with the recommendation for optical colonoscopy in appropriate patients [12]. Those patients with other comorbidities or risks may be better suited to undergo surveillance, as clinically indicated. Recent large trials in screening cohorts have reported lower rates of high-grade dysplasia and cancer in diminutive and small polyps, compared to earlier studies of mixed cohorts at greater risk for neoplasia. Specifically, in the Clinical Outcomes Research Initiative (CORI) database of 13,992 asymptomatic patients, the percentages of cancer, high-grade dysplasia, and tubulovillous histologies were 0, 0, and 1.2 %, respectively, in 1–5 mm polyps and 0.2, 0.8, and 4.4 %, respectively, in 6–9 mm polyps [27]. This is significantly less than corresponding results in earlier studies [28]. This size threshold was designed to balance the low risk of neoplasia of these smaller lesions within an interval growth rate of 5 years of surveillance, with the costs and morbidity of polypectomy [29].
C-RADS, Structured Reporting of CTC
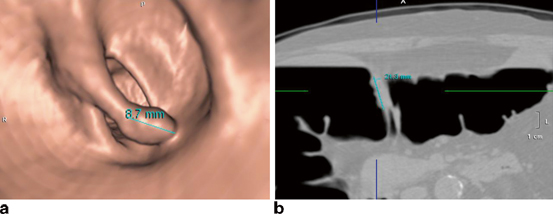
Consistency and standardization in reporting of findings at CTC have been aided by a reporting structure, called CTC reporting and data system (C-RADS), developed in 2005 [30]. This reporting structure was modeled after the successful development of breast imaging RADS (Bi-RADS) used in mammography. C-RADS describes how to report the individual colorectal findings based on lesion size, morphology, attenuation (density), and location. Namely, lesion size is defined as the linear long axis measurement of the polyp, with exclusion of the stalk if pedunculated, using a polyp window-level setting (e.g., image display that evaluates the polyp with surrounding air interface). For sessile or superficially elevated lesions, the long axis at the base of the lesion is measured (Fig. 5.1). Both 2D multiplanar (axial, sagittal, and coronal views) as well as 3D endoscopic (virtual endoscopic views) can be utilized to best portray maximal lesion size. Typically, 3D can give the best overall view of the polyp, but when the colon is tortuous, partially collapsed, or fluid filled with incomplete visualization of the lesion at 3D, the 2D views can play an important complimentary role and provide accurate measurement of lesion size. Morphological criteria include sessile (lesion base broader than lesion height by two times or more), pedunculated, flat (less than 3 mm of vertical height), and advanced mural lesion of cancer. Lesion attenuation or density describes whether the lesion is soft tissue or fatty. Polyps or masses have soft tissue attenuation, compared to the fat attenuation of lipomas or lipomatous ileocecal valves. With the use of high-density stool tagging agents, stool can be completely or predominantly tagged with barium or iodine agents. In contrast, the surface of polyps or cancers can have a linear or nodular high-density tagging in more than 40 % of lesions [31]. Lesion location uses the standardized six segments of rectum, sigmoid, descending colon, transverse colon, ascending colon, and cecum. Flexures are referred to generically, recognizing that correlation between flexures described at colonoscopy or based on anatomic definitions may defer from what is directly visualized at CTC. A key attribute of CTC is the ability to provide very accurate localization of a polyp or cancer within the colon, using the 3D transparency view (i.e., barium enema like visualization) as an overview of the colon with the specific finding(s) marked within it (Fig. 5.2). This can be very helpful to orient the gastroenterologist or colorectal surgeon to understand the colonic anatomy and location of lesions found at CTC.