FIT
Positivity (%)
Sensitivity (%)
Specificity (%)
Manufacturers’ quoted fecal hemoglobin cutoff (ng Hb/mL buffer)
A
6.4
29.8
96.7
50
B
11.0
30.5
92.9
40
C
22.3
53.2
81.8
10
D
24.1
56.0
82.0
40
E
35.0
59.6
70.2
50
F
46.8
73.4
58.8
25
The problem illustrated above can be easily rectified by converting the data in previous publications expressed in nanograms of Hb per milliliter of buffer to the recommended units using the formula:
μg Hb per g feces = (ng Hb per mL × mL buffer)/(mg feces collected).
Illustrations of how this can be done are shown in Tables 4.2 and 4.3.
Table 4.2
Fecal immunochemical test cutoff fecal hemoglobin concentration after unit standardization. (Courtesy of Jeffrey Lee, MD, Division of Gastroenterology, UCSF)
FIT brand | Cutoff fecal hemoglobin concentration reported in the literature or by manufacturer | Standardized fecal cutoff concentration hemoglobin (µg Hb/g feces) |
|---|---|---|
SENTiFOB | 100 ng/mL | 17 |
OC-Sensor/Micro/Diana | 100 ng/mL | 20 |
OC-light | 50 ng/mL | 10 |
FOB gold | 100 ng/mL | 17 |
Magstream 1000/Hem Sp | 20 ng/mL | 67 |
Monohaem | 0.02 mg/g | 20 |
FlexSure OBT (Hemoccult ICT) | 0.3 mg/g | 300 |
Ridascreen hemoglobin | 24.5 µg/g | 24.5 |
Table 4.3
Data supplied by the manufacturers of the four FIT evaluated by GMEC [48] to enable conversion between ng Hb/mL buffer and μg Hb/g feces
Analytical system | Sample weight (mg) | Buffer volume (mL) | Conversion factora |
|---|---|---|---|
HM-JACKarc | 2 | 2.0 | 1.00 |
NS-PLUS C15 | 10 | 1.9 | 0.19 |
OC-SENSOR DIANA | 10 | 2.0 | 0.20 |
FOB gold/BioMajesty | 10 | 1.7 | 0.17 |
Hb is not stable and degrades from the time it is released into the gastrointestinal tract. The rate of degradation depends on the chemical and microbiological composition of the feces, temperature, effectiveness of stabilizing bactericidal preservatives in the specimen collection device, and the period of time from the blood loss to analysis. The stability of Hb in the specimen collection devices needs to be characterized in a standardized way so that products can be compared and judgments can be made on their suitability for screening in different environments and under different climatic conditions.
As pointed out in a recent review in Gut and Liver [33], FIT analysis depends on antibodies binding to globin of Hb; thus, FIT are more susceptible to false-negative results if samples are not adequately preserved. In 2009, the Australian Commonwealth government had to temporarily suspend participation in the National Bowel Cancer Screening Program after problems were found in the buffer of the FIT kits that had been distributed between December 2008 and May 2009. The buffer in the FIT specimen collection device was not sufficiently effective at minimizing Hb degradation at the high temperatures found in the country during the summer period. As a result, the collection devices returned for analysis yielded a lower-than-expected proportion of positive results, with many participants receiving likely false-negative results. The potential for inaccurate FIT results during hot weather was investigated in a retrospective Italian study and showed f-Hb 17 % higher in the winter, when 13 % more cancers were detected [47]. Since 2011, companies have been actively enhancing the effectiveness of their preservative buffers, using antibacterial and stabilizing agents. An evaluation of quantitative FIT devices commissioned by the National Health Service (NHS) Bowel Cancer Screening Programme in England conducted in 2012/2013 showed that stability should now only present significant problems at very high ambient temperatures [48]. This report is supportive of recent product claims of increased stability. When product changes (enhancements) are introduced by manufacturers, it is important that customers are apprised of the changes and new product codes or designations are adopted to enable comparative clinical research to make valid observation about product performance over periods of time and in different countries.
Standards for FIT Processing and Development
FIT can be processed, developed, and interpreted at point of care (POC) by different types of health-care professionals or at centralized, automated, accredited laboratories by professionals in laboratory medicine experienced in using analytical systems and interpretation of numerical data. Most FIT in the USA are marketed for POC use. Automated analytical systems for FIT have many advantages [5, 49] over POC manual use and interpretation, including higher throughput and improved analytical accuracy and precision, while eliminating the potential for visual bias by observers. The person developing and interpreting manual tests often needs to wait for a prescribed period of time specified by the manufacturer after application of the feces in solution to the test cassette or strip before reading the test. This is prone to difficulties in a busy clinical setting and accurate interpretation of results for manually developed and interpreted fecal tests requires training and supervision, especially when interpreting borderline results [50–52] (Fig. 4.1). These facts should discourage those who might recommend patients screen themselves with over-the-counter FIT and “at home” development. Another advantage of laboratory analysis is that the user can select the f-Hb cutoff that will initiate further investigation, usually colonoscopy. To enable high analytical quality, we recommend the use of automated FIT systems and interpretation in accredited laboratories in preference to that of POC testing. We strongly recommend the use of laboratories accredited to internationally accepted standards, such as “ISO 15189: Medical Laboratories–Particular Requirements for Quality and Competence.” These will have comprehensive quality management systems in operation and will collect numerical quality indicators such as positivity rate, internal quality control data showing test result variation, imprecision and changes in bias over time, and performance in proficiency testing and external quality assessment.
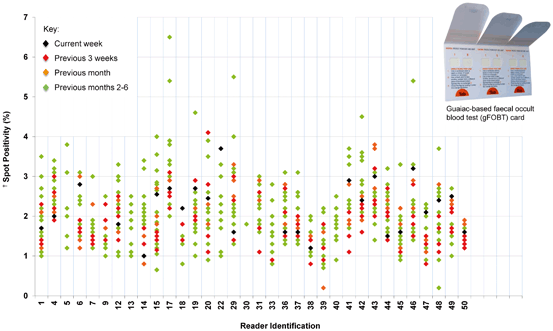

Fig. 4.1
Example of the variable spot positivity rate of well-trained, supervised, and monitored individuals reading guaiac-based fecal occult blood test (gFOBT) cards over a rolling 6-month period. († Spot positivity (%) calculated as (number of spots read as positive/number of spots read) for readers who have read at least 600 spots per week and where each “spot” is one of six windows (see card image))
If we recommend automated development and interpretation of FIT, we must be able to demonstrate the quality and accuracy of the recommended instruments for this task. To this end, the EWG has supported the evaluation of four quantitative FIT systems by the established Guildford Medical Device Evaluation Centre (GMEC) based in England [48]. At the commencement of the evaluation in December 2012, all systems (specimen collection devices and associated measurement instruments) that met criteria prescribed by the NHS Bowel Cancer Screening Programme were included in the evaluation. The following criteria for evaluation were required for systems to be suitable for population-based CRC screening:
The system provided a quantitative measurement of f-Hb.
The analysis could be automated.
The specimen collection device was suitable for home use.
For the evaluation, each manufacturer was asked to supply:
The analyzer
Consumables and reagents, including calibrators and controls
Training for the study laboratory professionals, scientists, and colleagues
Ongoing technical support
Four automated, quantitative FIT devices/analyzers were found to be eligible for evaluation, having met the simple criteria above:
FOB Gold NG, Sentinel CH. SpA, Italy/Biomajesty, Sysmex, UK
HM-JACKarc, Kyowa Medex Co. Ltd, Japan
NS-PLUS C15 Hb, Alfresa Pharma Corp, Italy
OC-SENSOR DIANA, Eiken Chemical Co. Ltd, Japan
The evaluation assessed the suitability of the specimen collection device for use in a population-screening program, including that of sending it through a postal system, performing to a consistently high analytical quality including analytical precision, suitable measurement range, adequate linearity, appropriate reporting units, and other performance characteristics. The suitability of the specimen collection devices was assessed by a large panel of users and is summarized in the report.
Summary and Conclusions
The idea that fecal tests are inferior to structural examinations has been promoted by US gastroenterology and endoscopy societies since 2000 when two screening colonoscopy studies were published in The New England Journal of Medicine [53, 54]. It was reinforced in 2008 with the publication of the “Joint guidelines from the American Cancer Society, the US Multi-society Task Force on Colorectal Cancer, and the American College of Radiology” and with the publication in 2009 of the American College of Gastroenterology “Guidelines for colorectal cancer screening” [1, 3]. Since 2000, screening rates for CRC have increased, but as of 2012 [55]:
1.
Only 65.1 % of US adults were up to date with CRC screening and 27.7 % had never been screened.
2.
The proportion of respondents who had never been screened was greater among those without insurance (55.0 %) and without a regular care provider (61.0 %) than among those with health insurance (24.0 %) and a regular care provider (23.5 %).
This information has led the Centers for Disease Control (CDC) to recommend promoting both fecal test for blood and colonoscopy as viable screening test options to increase CRC screening rates and reduce health disparities. The ACS and the National Colorectal Cancer Roundtable have advised physicians that fecal tests have been shown to decrease both incidence of and mortality from CRC and are reasonable screening test choices [56]. Endoscopic journals have published articles on CRC screening where experts have said: “Colonoscopy remains the dominant CRC screening strategy in the USA but is less effective at preventing right sided CRC than previously thought” and, “FIT has emerged as an effective low cost alternative to colonoscopy and is considered by some an equivalent or superior approach to screening as compared to colonoscopy.” [57] The American College of Physicians (ACP) in its Guidance Statement on Screening for CRC (2012) stated, “Shared decision making is important when selecting a screening test because the currently available colorectal cancer screening tests are believed to be similarly efficacious” [58]. Even a recently published Journal of the American Medical Association (JAMA) patient page on options for colorectal cancer screening states “Evidence does not yet support any one screening test over another, so in deciding which screening option is best for you, consider your personal health situation and talk with your doctor” [59].
This change in US institutional and professional society thinking about the value of screening tests other than optical colonoscopy is very welcome, although with acceptance comes responsibility. More well-designed and comparative, analytical, and clinical effectiveness studies must be government-funded. They are necessary to identify FIT products best qualified for population screening. Further standardization of the analytical and clinical performance characteristics of FIT is urgently needed. Those of us who promote the use of FIT for CRC screening must be sure that only the best FIT are chosen for population screening and that the conditions for collection and handling of samples, analysis, and interpretation of results are of the highest quality. We hope that publication of this chapter will help to achieve this goal.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree