Fig. 17.1
Quality and safety metrics in hematopoietic stem cell transplantation. Adopted from Rice and Bailey [31]
Donor Safety
As unrelated hematopoietic cell (HC) donation is only reserved for individuals >18 years of age worldwide, we will only review HC donation for related donors. There is no direct medical benefit from serving as a stem cell donor, though there is a psychosocial benefit of helping a sibling or close family member [32]. There are safety, quality, and ethical implications for both donors and recipients; consequently, various processes have been implemented by registries worldwide to minimize the risk to both parties. HLA-matched siblings are considered to be the best HC donors for both practical and biological purposes [33]. However, when the recipient is a child, potential sibling donors are often also children themselves. Today, more than one third of children undergoing allogeneic HSCT receive HC grafts from siblings under the age of 18 years [34, 35].
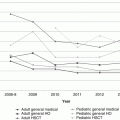
All stem cell sources, including bone marrow (BM), peripheral blood stem cells (PBSC), and cord blood (CB), can be obtained from pediatric donors, but BM-derived cells are the preferred source for many reasons such as a decreased risk of graft-versus-host disease (GVHD) [34, 35]. PBSC donation requires the donor to receive granulocyte colony-stimulating factor (G-CSF) and then undergo central venous catheter placement under anesthesia and apheresis. BM harvests are generally regarded as safe, but also require general anesthesia and may lead to pain at the harvest site. Although the BM and PBSC collection procedures differ greatly, the main symptoms experienced by BM and PBSC donors were similar: pain, fatigue, insomnia, local reactions, dizziness, anorexia, emesis, rash, and occasional fever or syncope [36] (Fig. 17.2). It is important to note there is no evidence that patients receiving G-CSF have an increased risk for cancer, autoimmune diseases, and/or stroke [37].
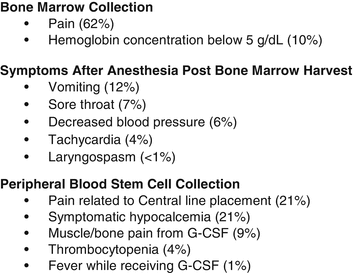

Fig. 17.2
Symptoms associated with hematopoietic cell collect (bone marrow and peripheral blood collection). Adopted from Styczynski [36]
In 2010, the American Academy of Pediatrics (AAP) released a policy statement on the use of children as donors. The statement outlined five conditions that the AAP recommends should be met in order for a minor to serve as a stem cell donor [38].
There is no medically equivalent HLA adult relative who is willing and able to donate.
A strong personal and positive relationship exists between the donor and recipient.
There is some likelihood that the recipient will benefit from transplantation.
The clinical, emotional, and psychosocial risks to the donor must be minimized and reasonable in relation to the benefits expected for both the donor and the recipient.
Parental permission and donor assent (when developmentally appropriate) must be obtained.
FACT and JACIE have specific guidelines for the collection of cellular products to protect the safety of donors during the process of HC collection. As donor safety is of utmost importance, checklists should be utilized to verify completion of the pre-procedure steps (e.g., testing for hemoglobinopathy, pregnancy, etc.) [39].
Coordination of Care
HSCT care is complex, involving multiple treatment modalities such as chemotherapy, radiation, and surgery; all need to be coordinated among different medical specialties. Treatment regimens can be time-intensive and debilitating and may result in serious, sometimes long-term, complications. Care coordination involves deliberately organizing patient care activities and sharing information between all of the participants involved in a patient’s care in order to provide safe and effective care. Simply put, the patient’s needs must be known and communicated to the right people at the right time, and this information must then be used to provide quality patient care [40–42]. There are three periods where comprehensive and effective care coordination is needed in the HSCT period: (a) pre-HSCT referral from the pediatrician or pediatric hematology-oncology physician, (b) during the peri-HSCT period where coordination between the HSCT team and other healthcare providers is needed, (c) in the post-HSCT period when the HSCT survivor is transitioned back to the PCP (Fig. 17.3).
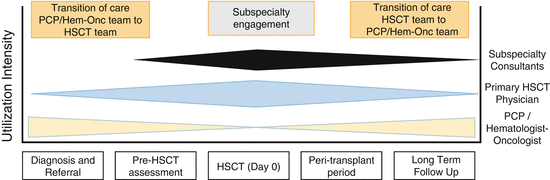

Fig. 17.3
Transitions of care in hematopoietic stem cell transplant (HSCT)
Pre-HSCT Care Coordination
Although the need for care coordination is clear, there are obstacles within our healthcare delivery system that must be overcome in order to provide thorough medical care. The Agency for Healthcare Research and Quality (AHRQ) acknowledges that our healthcare delivery system is often disjointed, and processes vary among primary care and specialty sites. Patients are often not certain of the reasons they are being referred from primary care to a specialist, how to make appointments, and what to do after seeing a specialist. Furthermore, specialists do not consistently receive clear reasons for the referral or adequate information on diagnostic evaluations that have been done prior to the referral [41].
The transfer of care of patients across clinical specialties is a complex process and is made even more challenging by cultural differences, individual expectations, and pressure from patients and families [43]. Oftentimes, referrals fail to meet the needs of either the initiating facility or the receiving provider [40, 43, 44]. Reasons for dissatisfaction include redundancies in the referral process, poor communication between physicians, the time required to write a referral note, and missing information in the referral letter or report [44]. Interestingly, pediatric specialists who received timely patient referral information reported providing optimal care twice as often as specialists who did not [45]. Unfortunately, in pediatric HSCT, there are no published reports relating to the referral process; this leaves opportunity for research and quality improvement in this area.
An effective referral mechanism ensures a close relationship between the initiating facility and the receiving facility. Successful subspecialty referrals require considerable coordination and interaction among the PCP, the subspecialist, and the patient, which may be challenging in the outpatient setting. Hysong et al. conducted a qualitative study to understand coordination breakdowns related to electronic referrals in an integrated healthcare system [46]. The authors examined work-system factors that affect the timely receipt of subspecialty care. Four overarching themes emerged: lack of an institutional referral policy, lack of standardization in certain referral procedures, ambiguity in roles and responsibilities, and inadequate resources to adapt and respond to referral requests effectively. Marked differences in PCPs’ and subspecialists’ communication styles and individual mental models of the referral processes likely precluded the development of a shared mental model to facilitate coordination and successful referral completion [46].
The AHRQ provides guidelines to improve care coordination with referrals. This approach can be utilized both in pre-HSCT referrals (from the PCP or hematologist/oncologist) or post-HSCT care back to the referring physician. AHRQ recommends that the referral process be designed by key stakeholders (e.g., referring oncology and BMT teams) to include all of the pertinent details necessary for effective and safe management of patients. As checklists have been shown to improve transitions of care through referrals [47], stakeholders should create a formalized checklist for patient referrals. These referral forms should include pertinent demographic, social, and medical information. AHRQ recommends that centers should not rely on patients to relay information, but should discuss and coordinate language barriers, verify that the patient understands the reason of the referral, and maintain a means to communicate progress throughout the HSCT process [41].
Currently, there are no metrics to measure referral effectiveness in HSCT. Physician teams should sample the number of referrals made over a specific time period (denominator) and calculate the percentage of referrals that included all relevant information (numerator). This could be tracked in real time, and QI methodology can then be used to close gaps in care.
Care Coordination in the Peri-transplant Period
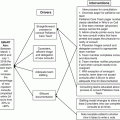
HSCT recipients are complex, and their care involves individuals from multiple specialties and services (Fig. 17.4). HSCT providers requesting subspecialist input in the hospitalized patient should provide the following information when requesting a consultation: (1) address the question that is asked, (2) whom to call with the response, (3) and the urgency of the consultation [48]. Physician consultation should be collaborative and multidisciplinary, and include patient and family engagement [49].
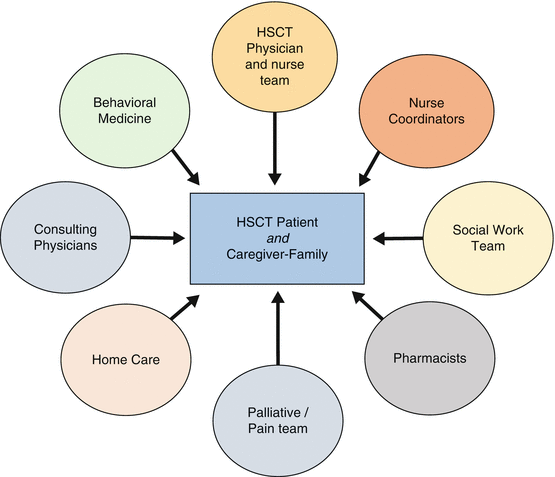

Fig. 17.4
Hematopoietic stem cell transplant (HSCT) care team in the peri-transplant period
HSCT nurse coordinators are instrumental in the overall management of HSCT patients. Nurse coordinators are involved throughout the entire HSCT course; they coordinate HLA typing between patients and potential donors, assure completeness of referral forms, verify insurance coverage, participate in the initial consultation with the transplant physician, schedule necessary procedures, and educate patients and families. Finally, nurse coordinators can assure adequate transition of care to the posttransplant setting and arrange for long-term follow-up [50].
Posttransplant Transition
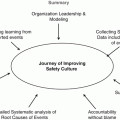
In the absence of relapse and/or active GVHD, most HSCT survivors are eventually able to transition to another set of providers. Ideally, this should be accomplished seamlessly with good communication. However, the vulnerabilities and complexities of such transitions of care have become evident, and medical and/or psychological crises may emerge or resurface among certain groups of patients who are at risk being lost in transition [51]. Oftentimes, HSCT survivors are accustomed to unique living arrangements and receive medical care within a complex healthcare delivery system which includes physicians, social workers, and other pediatric specialists. At times, the transition from the protective environment of the “bone marrow transplant medical home” can be difficult, as pediatric patients may rely on their caregivers well into their 30s and 40s [51–54]. The same requirements and steps described above should be used for the transition of care to the PCP. Cupit et al. recently reviewed the mechanisms to transition care of pediatric and adolescent young adult transition to adult healthcare providers [55]. Important considerations in HSCT transition are reviewed in Fig. 17.5.


Fig. 17.5
Considerations for successful transition of HSCT survivors. Adopted from Cubit et al. [55]
Survivorship and Long-Term Screening
Survivorship of HSCT begins on the day of transplantation [51]. There are few patients who have more complicated survivorship care than those who undergo HSCT in childhood. Quality pediatric HSCT care must include appropriate long-term follow-up to monitor and treat the complications associated with this intensive treatment. Many factors should be considered when determining the risk of long-term complications after HSCT and include the type and intensity of treatments received prior to the transplant, conditioning regimen, type of transplant and product, type and severity of GVHD, early complications, comorbid conditions, genetic factors, and lifestyle. HSCT survivorship tools are available to assist in appropriate screening for complications and include the Children’s Oncology Group, Long-Term Follow-Up Guidelines [56], and Pediatric Blood and Marrow Transplant Consortium Consensus Paper [57]. HSCT long-term follow-up teams should include, or have the ability to refer patients to, subspecialists with experience in the care of post-HSCT organ dysfunction (Table 17.1).
Table 17.1
Recommended organ function screening and late-effects multidisciplinary team members
Screening test | Minimum screening frequency | Multidisciplinary team member | |
|---|---|---|---|
Hematologic | Ferritin Hepatic function | Yearly | Hematologist with experience in chelation therapy |
Pulmonary | Pulmonary function tests (PFTs) | Every 6 months for first 2 years | Pulmonologist PFT lab Cardiologist with expertise in pulmonary hypertension |
Endocrine | Thyroid function Gonadal function Growth hormone | Yearly | Endocrinologist |
Renal | Blood pressure Renal function test Urinalysis Urine protein to creatinine ratio | Blood pressure assessment at each clinic visit Laboratory testing at day +80 and yearly post-HSCT | Nephrologist Pharmacist (to help adjust medications with renal dysfunction) |
Ocular | Ophthalmology evaluation | Yearly | Ophthalmologist with experience in the management of cataracts and ocular GVHD |
Cardiac | Echocardiography HgA1C Lipid screening | Yearly laboratory testing for metabolic syndrome Echo screening 1 and 5 years post-HSCT | Cardiologist with experience managing heart failure Echocardiography team |
Hematologic Complications
Many of the underlying diseases that necessitate HSCT require extensive blood product support prior to transplant, and all patients will require transfusion support in the pre-engraftment period. The cumulative effect of transfusion support is varying degrees of iron overload, which is associated with increased mortality before day 100, acute graft-versus-host disease, and blood stream infections [58–61]. While serum ferritin can be falsely elevated as an acute-phase reactant, elevated levels should serve as an indicator of iron overload and prompt further investigation and treatment [58, 62–64]. Multidisciplinary teams should include specialists able to manage chelation therapy if needed.
Pulmonary Complications
Pulmonary complications post BMT vary widely, including both restrictive and obstructive conditions due to acute or chronic GVHD, HSCT preparatory regimens, or infectious sequela. Furthermore, subclinical pulmonary dysfunction may be present in asymptomatic post-HSCT patients [65]. Serial pulmonary function tests (PFTs) are used to monitor survivors post-HSCT. A possible life-threatening pulmonary complication, bronchiolitis obliterans syndrome (BOS), typically occurs within the first few months to years after HSCT and leads to progressive pulmonary fibrosis and obstructive lung disease [66, 67]. In patients who develop BOS, providers should have a low threshold for obtaining echocardiographic screening for pulmonary hypertension [68]. Survivorship multidisciplinary teams should include a pulmonologist, PFT lab, and cardiologist with expertise in pulmonary hypertension.
Endocrine Complications
Endocrinopathies after BMT are common and can result from direct injury to endocrine organs or via disruption of the hypothalamic-pituitary axis. Endocrine dysfunction can include gonadal dysfunction, growth impairment, and thyroid dysfunction; [69–72] and screening for endocrine complications should include monitoring hormone levels and physical exam focusing on appropriate anthropometric and developmental measures including growth velocity and Tanner staging in children [73]. Transplant teams should work closely with endocrinology specialists in the screening and management of endocrine complications after HSCT.
Renal Complications
Three major categories of long-term renal complications are seen in BMT survivors: thrombotic microangiopathy, nephrotic syndrome, and idiopathic chronic kidney disease resulting from nephrotoxic medications used during transplant [74–76]. Screening for renal dysfunction during survivorship involves laboratory monitoring and blood pressure assessment. More comprehensive monitoring with glomerular filtration rate, ultrasonography, or renal biopsy may be warranted for survivors with established or suspected renal disease [56, 77]. Management of CKD during survivorship should focus on mitigating factors; discontinuation of nephrotoxic drugs and aggressive blood pressure control should be prioritized [78].
Ocular Complications
Cardiac Complications
Multiple cardiac complications may occur in survivors of HSCT. Echocardiographic findings of elevated right ventricular pressure (pulmonary hypertension) [68, 82], pericardial effusions [83–86], and left ventricular systolic dysfunction [87, 88] have been described in both adult and pediatric patients undergoing HSCT. In addition, HSCT survivors are at risk for early onset metabolic syndrome and coronary artery disease [75, 89–91]. Comprehensive teams should include access to echocardiography and a cardiology subspecialist with experience in the management of heart failure.
Standardized Process for HSCT Survivors
Long-term survivors of HSCT should receive comprehensive routine screening to insure early detection of late effects as it may lessen their long-term consequences. Transplant centers should create a reliable system to adequately screen HSCT survivors including compliance measurements of comprehensive follow-up. These data could be calculated over any specific time.
- (a)
Multidisciplinary teams should create standard screening protocols that are disease specific, and adaptable to previous treatments and HSCT complications (e.g., GVHD).
- (b)
Establish a mechanism to identify long-term follow-up patients and measure compliance with screening.
- (c)
Establish partnerships with subspecialists who have experience managing late HSCT complications.
- (d)
Barriers to follow-up should be identified. These include inconsistent scheduling of long-term follow-up patients, variability in physician practice with deviations from evidenced-based guidelines, and the lack of accountability and consistent tracking of BMT survivors.
- (e)
Test and develop intervention and processes to improve long-term follow-up rates. Examples of interventions include creation of a standardized follow-up checklist and processes to identify all survivors, as well as delineated roles and responsibilities for the post-BMT follow-up process.
- (f)
Establish a standardized mechanism for patients with abnormal post-HSCT screening results including referral to appropriate subspecialty care.
- (g)
It is important to continuously monitor adherence to the long-term survivor guidelines. Teams should encourage accountability to assure process compliance.
Posttransplant Quality of Life
HSCT is an area rich for the consideration of health-related quality of life (HRQOL) due to the profound impact it has on recipient’s physical and emotional well-being [92]. As therapies and associated supportive cares improve and patients experience increased survivorship, the idea of what constitutes a “successful” HSCT continues to evolve. It is not enough to cure the underlying disease; preservation of HRQOL including emotional, social, and physical well-being must also be of utmost importance.
HRQOL is a complex, multifaceted, and dynamic entity influenced by psychological and social functioning [93]. It has been endorsed by the World Health Organization as essential for measuring as a clinical outcome, separate from morbidity and mortality [94, 95]. For the purpose of evaluation, HRQOL is frequently divided into physical, social, and emotional/mental domains [96–101]. HRQOL is compromised even before patients undergo HSCT, likely due to prior treatment, underlying disease, and physical symptoms; it then worsens with the preparative regimen [93, 102, 103]. Although medical factors impact HRQOL, certain demographic factors are also potent predictors of HRQOL [103]. A 2009 review article by Tremolada et al. examined 47 studies on pediatric HSCT recipients relating to psychosocial sequelae and HRQOL; many studies showed that older age at HSCT, late effects, female gender, and more proximal time to transplant were risk factors found to be associated with poor HRQOL [93].
Recent studies show variable effects of age at HSCT on HRQOL [95, 103–107]. Patients who are older at the time of HSCT may experience more disruption to their daily lives; they may also more concretely anticipate future distress and prolonged illness than younger children [103]. Additionally, younger patients may not remember the HSCT experience as vividly as older patients do later in life [95]. Additionally, even after controlling for socioeconomic status, ethnicity impacts HRQOL with African-American children reporting the highest HRQOL, while children of Asian descent report the worst decline in HRQOL [103]. It is thought that the patients’ culture, behaviors, and values, in combination with their pre-HSCT experience, impact their expectations related to HSCT. Religion, spirituality, and social support, which are often culturally mediated, also impact HRQOL.
When compared to pediatric patients with hematologic malignancies who have been treated with chemotherapy alone, patients who have undergone HSCT have lower overall HRQOL scores [108]. Several studies have shown that these patients generally do not experience decreased social or emotional functioning post-HSCT; it is physical functioning that is most impaired [108–110]. Pediatric HSCT survivors also report more severe chronic health conditions later in life versus patients who received only chemotherapy [111–113]. As social and emotional functioning is essentially unchanged, they seem to adapt emotionally well to their limitations; it is the severity of physical dysfunction that appears to determine HRQOL in this patient population [114, 115].
In the absence of GVHD and late effects, HRQOL does ultimately improve post-HSCT. Studies have shown the timing of improvement to be variable, around 4–12 months post-HSCT [93, 103]. As early as 6 months post-HSCT, HRQOL can be comparable to, and sometimes better than, population normative data [93, 105]. Due to the lack of data investigating quality improvement in HRQOL, more research and investigation is needed.
Impact of Late Effects and GVHD
Post-HSCT late effects are common; they are well documented in the literature and negatively impact HRQOL [116–121]. Monitoring for late effects in pediatric patients post-HSCT is essential and is reviewed separately. Studies comparing childhood cancer survivors treated with chemotherapy alone versus those treated with HSCT demonstrate a significantly higher risk of late effects in those treated with HSCT [91, 114]. Additionally, physical dysfunction or limitations, psychological stress, and problems with social interactions are common in patients’ post-HSCT [91].
QOL in patients with late effects may vary by age, partly because some medical causes of impaired QOL, for example, gonadal failure or other organ damage, may not be fully realized until adulthood [114]. Therefore, QOL may be more preserved in children and adolescents but impaired once the patients reach adulthood.
Additionally, when patients experience GVHD, HRQOL declines. The Chronic GVHD Consortium has extensively studied the impact of chronic GVHD on HRQOL in adult post-HSCT patients [122]. Within HSCT recipients, patients with GVHD have significantly worse HRQOL, both clinically and statistically, versus those without GVHD [123].
Challenges to Measuring HRQOL in Pediatric Patients
The National Institutes of Health (NIH) Consensus Conference on Criteria for Clinical Trials in chronic GVHD recommended the use of health-related QOL (HRQOL) tools in adult patients for a standardized measure of the impact of disease burden and patient outcomes [124–126]. A unidimensional measure of global HRQOL has also been developed as part of the Patient-Reported Outcomes Measurement Information System (PROMIS) project [127]. Despite these efforts, there have been no formal recommendations for a pediatric-specific HRQOL tool.
The use of varying HRQOL surveys for assessment in pediatric patients, along with a wide spectrum of diseases and a limited number of patients, leads to inconsistent results among pediatric HRQOL studies [93, 123]. Another major challenge of measuring HRQOL in pediatric patients is that these patients are dynamic; physical, emotional, social, and cognitive development changes occur with time and must be accounted for in a measurement tool [128].
Parent-Patient Concordance
In addition to the lack of age-appropriate QOL measures, another historic limitation in the evaluation of pediatric HR QOL was the belief that children did not have the ability to reflect on their own QOL, necessitating parents as proxy QOL raters. However, HRQOL ratings differ between patients and parents, perhaps due to information variance, the unequal understanding of and/or access to information, effects of age on processing and interpretation of information, and the child’s ability to understand the gravity of both the underlying diagnosis and treatment modalities [92]. Criterion variance, the difference in weight given by each to the available information, also impacts proxy HRQOL scores; parents may compare the child’s current health status to his/her potential future status, the health status of siblings or peers [92].
New Tools
Using the Child Health Rating Inventories (CHRIs) tool, Rodday et al. created child, adolescent, and parent surveys to evaluate seven HRQOL modalities in pediatric HSCT recipients: physical health, mental health, family life, friendship, self-confidence, fun, and life enjoyment [129, 130]. Its brevity yields simplicity, ease of use, and decreased responder burden, making it an ideal tool for which to screen patients who may require more in-depth HRQOL evaluation.
Lawitschka et al. developed the PedsQL Stem Cell Transplant module, an HSCT-specific tool for HRQOL assessment in children and adolescents [128]. The instrument was based on the PedsQL Generic Core Scale [131, 132]. It contains the following domains: pain and hurt, fatigue, nausea, worry or anxiety about disease and/or treatment, nutritional problems, thinking and remembering, communication about disease and/or treatment, and chronic GVHD symptoms. A multicenter validation trial is currently underway.
Selected Topics in HSCT Quality and Safety
Bloodstream Infections (BSIs)
HSCT patients are at increased risk for developing bacterial bloodstream infections (BSIs), which are among the most serious infectious complications and a known cause of increased non-relapse mortality (NRM) in this patient population [133–135].
BSIs in the healthcare setting are classified as primary BSI, related to either a central venous line (CVL) or other hospital-acquired source, or secondary BSI, a bacteremia related to another site of infection (e.g., abscess or pneumonia) [136]. Thus, unless an alternative source is identified, all BSIs in patients with a CVL are considered central line-associated bloodstream infections (CLABSIs). CLABSIs are serious complications in HSCT recipients and lead to prolonged hospitalization, intensive care admissions, and antibiotic treatment [133, 134, 137]. Some patients with CVLs experience BSIs that do not arise from the catheter, but rather originate from translocation of bacteria through non-intact oral and gut mucosa [136, 138]. To address this type of BSI, the Centers for Disease Control and Prevention defined a specific CLABSI type known as “mucosal barrier injury laboratory-confirmed bloodstream infection” (MBI-LCBI) on the basis of literature review and expert opinion. In 2013, the MBI-LCBI definition was integrated into the National Healthcare Safety Network (NHSN) methods for primary BSI surveillance to identify a subset of BSIs reported as CLABSIs that were likely related to MBI in the mouth and gut and not the presence of the CVL itself and that occurred most frequently in patients with neutropenia [136]. Currently, primary BSIs in patients with a CVL are defined as “laboratory-confirmed bloodstream infection (LCBI)” and subcategorized as “CLABSI” or “MBI-LCBI” [139]. Inherent to this distinction is emerging evidence showing that improved CVL maintenance is effective at reducing CLABSI rates [140–142], but not in preventing MBI-LCBIs [143].
Catheter Care Bundles
Catheter care bundles, for both CVL insertion and maintenance, consist of a standard combination of evidence-based interventions that have been shown to be effective in preventing CLABSIs and improving patient outcomes [144, 145]. Germane bundle components include performance of hand hygiene, full-barrier precautions including the use of sterile technique and chlorhexidine cleansing during insertion, and proper procedures for CVL access, manipulation, and dressing changes. Standardization of bundle elements coupled with systematic implementation and compliance has been shown to significantly reduce CLABSI rates across multiple studies of pediatric oncology and HSCT patients [146–150]. Best practice bundle implementation with particular focus on maintenance strategies also reduces CLABSI rates in the ambulatory setting [146, 151]. As part of a multicenter quality improvement initiative, 32 pediatric hematology/oncology and bone marrow transplant centers across the USA implemented a standardized bundle of CVL care practices. Average compliance with the CVL care bundle across the institutions was greater than 80% during the study period, and the collaboration demonstrated a decrease in CLABSI rates from 2.85 CLABSI/1000 CVL days to 2.04 CLABSI/1000 CVL days, a reduction of 28% (RR, 0.71; 95% CI, 0.55–0.92) [152]. This multi-institutional collaborative improvement effort succeeded at reducing CLABSI rates through standardized CVL bundle care in immunocompromised patients. In a recent study from Memorial Sloan Kettering Cancer Center (MSKCC), rates of hospital-acquired CLABSI in high-risk adult patients, including HSCT recipients, after implementing the use of a disinfection cap were 2.3/1000 days, representing 34% decrease from previous periods and resulted in substantial cost savings [153].
Microsystem Stress
Microsystem stress can arise from high patient volumes and acuity and is associated with increased mortality, failure-to-rescue rates, and increased nurse burnout [154, 155]. Additionally, increased workload can influence the provider’s decision to perform various procedures [156], reduce patient satisfaction [157], decrease communication between nurses and patients [158], and decrease collaboration between providers [159].
Dandoy et al. evaluated the effects of microsystem stress on CLABSI rates. Over a 1-year period of time, their institution saw increased stressors to their healthcare delivery system: the average daily float nurse hours increased nearly 400%, average daily census increased 30%, the number of new relapsed or refractory patients increased 200%, and the percentage of nurses with less than 1 license year increased 100%. Corresponding with these acute stressors, the CLABSI rate increased from 1 to 2 CLABSIs/1000 CVL days. The multidisciplinary team identified key processes to mitigate potential drivers to the increased CLABSI rate and, through small tests of change, implemented a standardized process for daily hygiene, increased awareness of high-risk patients with CLABSI, improved education/assistance for nurses performing high-risk central venous catheter procedures, and developed a system to improve allocation of resources to de-escalate system stress. After implementation of the interventions, the CLABSI rate decreased nearly 70% (0.39 CLABSIs/1000 CVL days).
Microsystem stress, caused by increased census and acuity, can be extended to physicians as well. Neuraz et al. performed a multicenter analysis evaluating workload and mortality in eight adult ICUs. The risk of death was increased by 2.0 (95% CI, 1.3–3.2) when the patient-to-physician ratio exceeded 14. High patient turnover (adjusted relative risk, 5.6 [2.0–15.0]) and the volume of life-sustaining procedures performed by staff (adjusted relative risk, 5.9 [4.3–7.9]) were also associated with increased mortality [160]. Additional studies, including hospitalists, intensivists, and surgeons, report that excessive attending physician workload has a negative impact on patient care [161–163]. These studies suggest that hospitals should provide mechanisms to provide greater staffing assistance and systems responsive to acuity and census fluctuations to improve safety and quality of care.
Cardiac Monitor Alarms and Alarm Fatigue
Alarm fatigue, the lack of response due to excessive numbers of alarms resulting in sensory overload, can create desensitization and result in missed alarms [164, 165]. In addition, high alarm rates can lead to decreased response to alarms, discomfort for patient families, and unnecessary resource utilization [166]. Due to the risk for acute decompensation, cardiac monitor alarms are frequently utilized in pediatric HSCT inpatients.
Dandoy et al. determined the impact of implementation of a standardized cardiac monitor care process on the rate of cardiac monitor alarms, and alarm fatigue in the Bone Marrow Transplant Unit at Cincinnati Children’s Hospital Medical Center [166]. The team measured the number of alarms per monitored day on patients. The cardiac monitor care process was developed through evaluation of the existing literature, and through Plan-Do-Study-Act testing. The standardized process included measurement of four components:
- (a)
Age-appropriate parameters for patients upon placement on a cardiac monitor
- (b)
Daily electrode changes
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree