Fig. 7.1
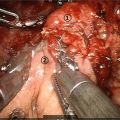
Dissection around the pylorus ring; the right gastric artery is dissected by the root, and the first pyloric branch is dissected along the lesser curvature of the stomach. The first pyloric branch of the right gastroepiploic artery is also dissected along the greater curvature of the stomach
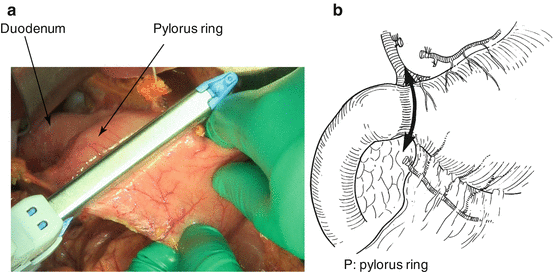
Fig. 7.2
(a) Resection site of the stomach in PrPD. (b) The stomach is divided just adjacent the pylorus ring
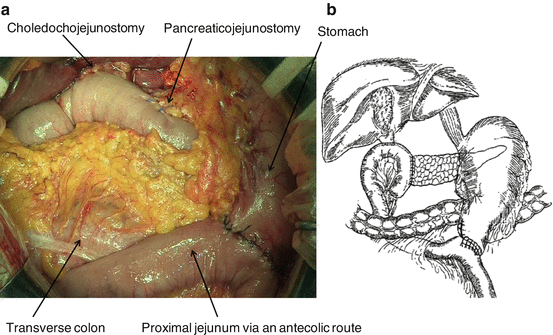
Fig. 7.3
(a) Gastrojejunostomy in PrPD is performed by a two layer anastomosis via an antecolic route. (b) The reconstruction is performed by conventional Billroth II reconstruction
7.3 Which Is Better, PD, PpPD, or PrPD?
There are three types of procedures for periampullary tumors or pancreatic head cancer as follows: PD, PpPD, and PrPD. However, it remains still controversial which is better procedure to reduce postoperative complication or improve long-term outcomes among three procedures.
Five RCTs comparing PD with PpPD have been conducted regarding short-term outcomes and long-term outcomes (Table 7.1) [10–14]. These studies have demonstrated that PpPD can facilitate a better nutritional status and more favorable quality of life without differences in mortality, morbidity, or oncologic outcomes, compared to PD. Regarding short-term outcomes, five RCTs revealed no significant differences in the incidence of postoperative complications such as pancreatic fistula, intra-abdominal abscess, or intra-abdominal bleeding between PD and PpPD. Two meta-analyses suggested that there were no significant differences in postoperative complications between PD and PpPD [15, 16]. PD had a mortality range of 0–7%, while the mortality due to PpPD ranged from 3 to 11% in the five RCTs. There were no significant differences between the two procedures regarding mortality. Therefore, the two procedures were equally effective for periampullary tumors in terms of morbidity and mortality.
Table 7.1
Summary of five randomized controlled trials comparing PD to PpPD
Authors | Years | Variable | Sample size | DGE (%) | Definition of DGEa | PFb (%) | Mortality (%) |
|---|---|---|---|---|---|---|---|
Lin et al. [10] | 1999 | PD | 16 | 7 | The nasogastric tube is left in place for 10 days or more plus one of the following: (1) Emesis after removal of nasogastric tube, (2) Reinsertion of nasogastric tube, or (3) Failure to progress with diet | 0 | 7 |
PpPD | 15 | 38 | 13 | 0 | |||
Seiler et al. [11] | 2000 | PD | 40 | 45 | A persistent drainage via the nasogastric tube of more than 500 ml/day for at least 5 days after surgery, or recurrent vomiting in combination with edema of the gastrojejunostomy or duodenojejunostomy and proximal dilatation on contrast radiography | 2 | 5 |
PpPD | 370 | 37 | 3 | 3 | |||
Tran et al. [12] | 2004 | PD | 83 | 23 | Gastric stasis requiring nasogastric intubation for 10 days or more or the inability to tolerate a regular diet on the 14th postoperative day | 14 | 7 |
PpPD | 87 | 22 | 13 | 3 | |||
Seiler et al. [13] | 2005 | PD | 66 | 45 | A persistent drainage via the nasogastric tube of more than 500 ml/day for at least 5 days after surgery, or recurrent vomiting in combination with edema of the gastrojejunostomy or duodenojejunostomy and proximal dilatation on contrast radiography | 2 | 3 |
PpPD | 64 | 31 | 3 | 2 | |||
Lin et al. [14] | 2005 | PD | 19 | 0 | The nasogastric tube is left in place for 10 days or more plus one of the following: (1) Emesis after removal of nasogastric tube, (2) Reinsertion of nasogastric tube, or (3) Failure to progress with diet | 5 | 11 |
PpPD | 14 | 43 | 7 | 7 |
Regarding long-term outcome between PD and PpPD, two RCTs have reported that body weight change and QOL did not exhibit significant differences between the two procedures [12, 13]. Seiler et al. used the Sickness Impact Profile (SIP), a standard questionnaire that assesses various physical, psychological, and social functions to compare the long-term QOL between PD and PpPD [13]. They reported that the capacity to work at 6 months after surgery was better after PpPD (77%) than after PD (56%), although the postoperative QOL did not significantly differ between the two procedures [13]. Regarding the survival rate between patients treated by PD and PpPD, Seiler et al. and Tran et al. in their RCTs reported that the long-term survival and disease-free survival were not significantly different between the two procedures [12, 13]. The two meta-analyses also suggested that there were also no significant differences in long-term survival between the two procedures. Therefore, the two procedures offered similar survival for periampullary tumors [15, 16].
7.4 The Impact of Pylorus-Resecting Pancreaticoduodenectomy (PrPD)
DGE is a persistent and frustrating complication in pancreaticoduodenectomy. Regarding DGE between PD and PpPD, Lin et al. reported that DGE occurred more frequently with PpPD (6 of 14 patients; 42.8%) than PD (0 of 19 patients; 0%) (P < 0.05) [14]. However, the sample size of this RCT was small (n = 33) and the RCT was limited to patients with pancreatic head cancer. On the other hand, Tran et al. reported in their RCT that no significant difference between PpPD (19 of 85 patients; 22%) and PD (18 of 80 patients; 23%) regarding the incidence of DGE [12] was observed. Seiler et al. also reported that there was no significant difference between PpPD (30 of 66 patients; 45%) and PD (20 of 64 patients; 31%) regarding the incidence of DGE [13]. The pathogenesis of DGE after PpPD has been thought to include several factors, such as (1) antroduodenal ischemia [31, 32], (2) gastric atony caused by vagotomy [33], (3) pylorospasm [34–36], (4) the absence of gastrointestinal hormones [37], (5) gastric dysrhythmia secondary to other complications such as a pancreatic fistula [38–40], and (6) antroduodenal congestion [41]. In particular, DGE after PpPD has been attributed to denervation and devascularization of the pyloric ring due to pylorospasms caused by surgical injuries of the vagus nerves innervating the pyloric ring. In PrPD, the stomach is divided adjacent to the pylorus ring and more than 95% of the stomach is preserved, although the pylorus ring is resected. PrPD was designed with expectation in maintaining the favorable stomach pooling ability and reducing the incidence of DGE compared to PpPD [30]. The technical modification of resecting pylorus ring may provide a simple and effective method to prevent the incidence of DGE.
Table 7.2 shows summary for comparative study between PpPD and PrPD (SSPPD) [30, 42–46]. There are two RCTs and five retrospective studies which compared PpPD to PrPD (SSPPD) based on DGE defined by the international study group of pancreatic surgery (ISGPS) [47]. RCT which compared PpPD with PrPD demonstrated that PrPD (4.5%) resulted in a significant reduction in the incidence of DGE compared with PpPD (17.2%) (P = 0.0244) [30]. As the objective data for DGE in this study, the 13C-acetate breath test, which is a simple and excellent indirect test to reflect gastric emptying, was examined. T max (the time of peak 13CO2 content after the administration of 13C-acetate) was reported to be a more useful marker reflecting gastric emptying. The time to peak 13CO2 content in the 13C-acetate breath test at 1, 3, and 6 months postoperatively to evaluate gastric emptying was significantly delayed in the PpPD group compared with the PrPD group [30]. On the other hand, another RCT by Matsumoto et al. reported that the incidence of DGE was 20% with PpPD and 12% with SSPPD (P = 0.414) [46]. The RCT demonstrated that no significant difference in the incidence of DGE was observed between PpPD and SSPPD. Matsumoto et al. discussed that this discrepancy between two RCTS was due to differences in the study subjects. So, in their study, pancreatic cancer was excluded because patients with pancreatic cancer underwent a more invasive surgery including portal vein resection and regional lymph node dissection than other benign or low-grade malignant lesions. However, Fujii et al. reported that SSPPD offers better perioperative and long-term outcomes for pancreatic cancer compared to PpPD [44]. Two meta-analyses comparing PrPD with PpPD reported that PrPD resulted in a significant reduction of the incidence of DGE compared to PpPD [48, 49]. As a modified anastomosis to prevent occurrence of DGE in SSPPD, Nakamura et al. demonstrated the greater curvature side-to-side anastomosis of gastrojejunostomy [50]. In the side-to-side anastomosis, the jejunal loop is anastomosed to the greater curvature 5–10 cm proximal to the closed gastric stump, and the anastomosis is just the greater curvature, not the anterior nor the posterior wall of the stomach. The study reported that the incidence of DGE in side-to-side anastomosis was 2.5% in side-to-side anastomosis and 21.3% in end-to-side anastomosis (P = 0.0002). It was concluded that the greater curvature side-to-side anastomosis of gastrojejunostomy significantly reduced the incidence of DGE compared to the gastric stump-to-jejunal end-to-side anastomosis in SSPPD. Now, PROPP study which compares PrPD to PpPD by RCT with sample size for 89 patients per group has been proceeding by Hackert et al. in Germany [51].
Table 7.2
Summary of comparative studies between PpPD and PrPD (SSPPD)
Authors | Study design | Years | Variable | Sample size | Definition of DGEa | DGE (%) | P value |
|---|---|---|---|---|---|---|---|
Kurahara et al. [42] | Retrospective study | 2010 | PpPD | 48 | ISGPSb | 34.8 | NS |
SSPPD | 64 | 13.0 | |||||
Kawai et al. [30] | Randomized controlled trial | 2011 | PpPD | 64 | ISGPSb | 17.2 | 0.024 |
PrPD | 66 | 4.5 | |||||
Fujii et al. [43] | Retrospective study | 2012 | PpPD | 33 | ISGPSb | 27.3c | 0.0012 |
SSPPD | 56 | 5.8c | |||||
Nanashima et al. [44]
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|