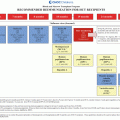
Fig. 8.1
Mechanisms of pulmonary dysfunction and/or growth by hematopoietic stem cell transplantation (HSCT). BO bronchiolitis obliterans, BOOP bronchiolitis obliterans organizing pneumonia, DAH diffuse alveolar hemorrhage, IPS idiopathic pneumonia syndroma
In other words, it is often difficult to discern which factors have the causative role in chronic lung disease. Both timing of the transplant and age at transplant are potential risk factors for late onset pulmonary morbidities. That is, those patients who received HSCT after two to three remissions (and therefore, most likely more chemotherapy incurring lung toxicity) had higher rates of these complications than those survivors transplanted in first remission [31]. Further, studies report that children transplanted at an older age had poorer pulmonary function testing values than those transplanted at a younger age [31]. Lastly, patients who have received prolonged immunosuppression for chronic graft versus host disease are at particular risk [31].
Patients undergoing total body irradiation as part of their conditioning regimen for stem cell transplantation have a high incidence of pulmonary late effects [21, 31]. As discussed, busulfan, carmustine, bleomycin and cyclophosphamide, all associated with pulmonary late sequelae, are known to cause pneumonitis and fibrosis after transplantation and this risk is increased in those who had previous radiation exposure or total-body irradiation for their preparative regimen.
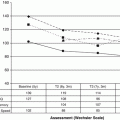
Longitudinal data in survivors of HSCT during childhood demonstrate that there is a decline in lung volume and diffusing capacity from pretransplant for 3–6 months post transplant, partial recovery for 1–2 years, and then stabilization for up to 10 years post transplant. The most common finding on pulmonary function testing is restrictive lung disease, sometimes associated with a decrease in DLCO [2]. In a cohort of 89 childhood cancer survivors post allogeneic HSCT, Inaba reported progressive worsening of pulmonary function as measured by forced mid-expiratory flow (25–50 %), residual volume, total lung capacity (TLC), and DLCO. Obstructive lung disease is also observed in HSCT survivors. For example, Bruno reported that in a series of 80 children treated with allogeneic HSCT, mean FEV1/FVC values of less than 60 % of predicted were observed in patients whose chronic graft versus host disease persisted 5 years after transplant.
8.5 Thoracic Surgery and Lung Damage
A mainstay of therapy for the treatment of pulmonary metastases (particularly metastatic osteosarcoma) is surgical resection. Although children are more adaptive to lung resection than adults, a study of adult childhood cancer survivors more than 30 years post resection, demonstrated increased rates of hypertrophy or hyperinflation of the remaining lung as a compensatory effect for the long-term loss in lung volume. Radiation exposure heightens the risk of pulmonary late effects when combined with surgical resection. In a Dutch cohort of childhood cancer survivors exposed to pulmonary toxic therapy, those with a history of surgical resection combined with radiation exposure had the highest increased risk of long-term pulmonary function impairment [5].
8.6 Other Risk Factors for Pulmonary Late Effects
In addition to therapeutic exposure, children with cancer may have other risk factors that predispose them to lung disease. These include underlying asthma or chronic obstructive lung disease, infection, cigarette or marijuana use, and exposure to environmental toxins. It is not known how the aging process and associated decline in lung function will affect survivors who had lung injury during cancer therapy in combination with other co-morbid heart or lung problems. This is an area prime for future research.
8.7 Detection, Screening and Management
Care of survivors at risk for pulmonary toxicity should include an annual medical history and physical examination, and careful review of a patient’s treatment summary. Chest X-ray and pulmonary function testing (including DLCO and spirometry) are recommended at entry into a long-term follow-up program and then as clinically indicated in patients with abnormal results or clinical symptoms (see Table 8.1).
Table 8.1
Pulmonary late effects and guidelines for surveillance a
Exposure | Potential Late Effect(s) | Risk Factors | Greatest Risk Factors | Pulmonary Health Evaluation and Counseling |
|---|---|---|---|---|
Bleomycin | Interstitial pneumonitis Pulmonary fibrosis ARDS (rare) | Host Factors Younger age at treatment Treatment Factors Higher cumulative dose Combined with: – Busulfan – BCNU – CCNU Medical Conditions Renal dysfunction High dose oxygen support such as during general anesthesia Health Behaviors Smoking | Treatment Factors Cumulative dose ≥400 U/m2 (injury observed in doses 60–100 U/m2) | Evaluation Yearly pulmonary exam Screening Chest X-ray (CXR) Pulmonary function tests (PFTs; including DLCO and spirometry) Baseline at entry into long-term follow-up. Repeat as clinically indicated in patients with abnormal results or progressive pulmonary dysfunction. Counseling Tobacco avoidance/smoking cessation To SCUBA dive, patients should have medical clearance from a pulmonologist For Bleomycin exposed survivors, notify healthcare providers of history of exposure and risk of worsening fibrosis with high dose oxygen exposure such as during general anesthesia. |
Alkylators – Busulfan – Carmustine (BCNU) – Lomustine (CCNU) | Pulmonary fibrosis | Treatment Factors Higher cumulative doses Combined with bleomycin Medical Conditions Atopic history Health Behaviors Smoking | Treatment Factors BCNU ≥600 mg/m2 Busulfan ≥500 mg (transplant doses) Combined with: – Chest radiation – TBI | Other considerations In survivors with abnormal PFTs and/or CXR, consider repeat evaluation prior to general anesthesia Refer to pulmonologist in survivors with symptomatic or progressive pulmonary dysfunction. Yearly influenza vaccines. Appropriate pneumococcal vaccination. |
Radiation – Chest – Whole lung – Mediastinum – Axilla – Mini-mantle – Extended mantle – Total Body Irradiation (TBI) | Pulmonary fibrosis Interstitial pneumonitis Restrictive lung disease – Growth abnormalities – Obstructive lung disease | Host Factors Younger age at irradiation Treatment Factors Radiation dose ≥10 Gy Chest radiation combined with TBI Radiation combined with: – Bleomycin – Busulfan – Carmustine (BCNU) – Lomustine (CCNU) – Radiomimetic chemotherapy (e.g., doxorubicin, dactinomycin) Medical Conditions Atopic history Health Behaviors Smoking | Treatment Factors Radiation dose ≥15 Gy TBI ≥6 Gy in single fraction TBI ≥12 Gy fractionated | |
Stem Cell Transplant – With any history of chronic GVHD | Bronchiolitis obliterans Chronic bronchitis Bronchiectasis | Treatment Factors Chest radiation
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|