Pulmonary embolism (PE) is the most dangerous complication of venous thrombosis. Objectively confirmed PE is a potentially life-threatening complication of critical illness. In medical-surgical critically ill patients, signs and symptoms are nonspecific, the clinical pretest probability may be low, and diagnostic tests may not be done or may yield ambiguous results. Therefore, PE is often undiagnosed and untreated; autopsy findings indicate that PE is common in intensive care unit (ICU) decedents. PE may delay weaning from mechanical ventilation, increase the length of stay in ICU and hospital, and increase the risk of death. More research is needed on PE in medical-surgical ICU patients including issues on the epidemiologic aspects of PE (including risk factors and attributable morbidity and mortality) and treatment of PE (including pharmacologic and nonpharmacologic approaches, determinants of treatment variation, and predictors of PE outcome adjusted for treatment).
Venous thromboembolism during critical illness
Venous thromboembolism (VTE), which includes deep vein thrombosis (DVT) and pulmonary embolism (PE), is a common complication of critical illness. Most thrombi are asymptomatic and are confined to the deep veins of the calf. However, with time, 20% to 30% of untreated calf vein thrombi extend proximally into the thigh, where, if untreated, they pose a 40% to 50% risk of PE. Early studies of the natural history of PE suggest that untreated PE has a 25% mortality rate.
Although DVT has potentially serious consequences, it is often unrecognized in critically ill patients. In an observational study of 100 patients admitted to the intensive care unit (ICU), lower limb Doppler ultrasonography was performed twice weekly. In these patients, whose type of thromboprophylaxis was selected by physicians, DVT was diagnosed in 32% of those receiving no intervention, in 40% of those receiving subcutaneous unfractionated heparin (UFH), and in 33% of those receiving mechanical prophylaxis (antiembolic stockings or pneumatic compression devices). Concern about undiagnosed VTE in the medical-surgical ICU setting is underscored by studies showing that 10% to 100% of DVTs found by ultrasonographic screening were not detected on physical examination. In a study of lower limb ultrasonographic screening done twice weekly in medical-surgical ICU patients, proximal DVT occurred in 25 of 261 (9.6%) patients during the ICU stay and in 4 additional patients after ICU discharge, accounting for a total of 29 of 261 (11.1%) patients. All but one case of DVT was clinically unsuspected, and all cases occurred despite routine heparin thromboprophylaxis.
Clinically unsuspected PE may also have catastrophic consequences in ICU patients. Mechanically ventilated patients who have sudden hypoxemia, hypotension, or tachycardia may have undetected PE. PE may also contribute to difficulty in weaning patients from mechanical ventilation. In ICU patients with impaired cardiopulmonary reserve, even a small PE might have severe or fatal consequences. In one study, 13 of 34 (38.2%) critically ill patients with known DVT and no symptoms of PE had PE diagnosed by ventilation-perfusion scans. In an autopsy study, PE was found in 59 of 404 (15%) hospitalized patients. In another autopsy study, PE was unsuspected during antemortem in 14 of 20 (70%) patients who died of PE. In a 25-year longitudinal study, 9% of patients had PE at autopsy, but in 84% of these patients, the antemortem diagnosis was missed.
In summary, DVT and PE are common in ICU patients; PE remains one of the most common unsuspected autopsy findings in critically ill patients.
Thromboprophylaxis in medical-surgical ICU patients
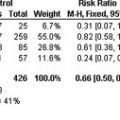
Given the high prevalence of VTE and the potential morbidity and mortality associated with PE, it follows that if VTE prophylaxis is effective, it should be used in the ICU. The beneficial effect of subcutaneous UFH to prevent VTE has long been established. In 2 meta-analyses of trials enrolling more than 8000 patients who underwent general surgery, UFH resulted in a 60% to 70% relative risk reduction for DVT and fatal PE. Strategies to prevent VTE are of proven benefit in multiple patient populations, are widely available, and have a low risk of toxicity.
However, only 2 randomized clinical trials have compared active thromboprophylaxis with placebo in critically ill medical-surgical patients. One double-blind randomized trial allocated 199 medical-surgical ICU patients to a twice-daily subcutaneous administration of UFH, 5000 IU, or placebo. Using serial fibrinogen leg scanning for 5 days, the DVT rates were found to be 13% in the UFH group and 29% in the placebo group (relative risk reduction being 0.65). Rates of bleeding, PE, and mortality were not reported. Subsequent demonstration that leg scanning is not a reliable diagnostic test for DVT limits the utility of this study. In a multicenter trial, Fraisse and colleagues randomized 223 patients mechanically ventilated for chronic obstructive pulmonary disease to a once-daily administration of the low-molecular-weight heparin (LMWH), nadroparin, or placebo. Patients had weekly ultrasonography, and venography was recommended at 21 days or earlier if results from ultrasonography were positive or nondiagnostic. The proportion of patients developing DVT was 16% in the nadroparin group and 28% in the placebo group (relative risk reduction being 0.45). There was a trend toward increased overall bleeding (25 vs 18 patients, P = .18) and major hemorrhage (6 vs 3 patients, P = .28) in the nadroparin group. PE was not systematically evaluated; 8 patients in each group died ( P = .72), but autopsies were not performed.
In summary, the beneficial effect of thromboprophylaxis on DVT in medical-surgical patients has been documented. However, whether thromboprophylaxis decreases PE rates alone has not been directly determined in randomized trials because of the small sample sizes of the available trials and because only few patients were diagnosed with PE.
Thromboprophylaxis in medical-surgical ICU patients
Given the high prevalence of VTE and the potential morbidity and mortality associated with PE, it follows that if VTE prophylaxis is effective, it should be used in the ICU. The beneficial effect of subcutaneous UFH to prevent VTE has long been established. In 2 meta-analyses of trials enrolling more than 8000 patients who underwent general surgery, UFH resulted in a 60% to 70% relative risk reduction for DVT and fatal PE. Strategies to prevent VTE are of proven benefit in multiple patient populations, are widely available, and have a low risk of toxicity.
However, only 2 randomized clinical trials have compared active thromboprophylaxis with placebo in critically ill medical-surgical patients. One double-blind randomized trial allocated 199 medical-surgical ICU patients to a twice-daily subcutaneous administration of UFH, 5000 IU, or placebo. Using serial fibrinogen leg scanning for 5 days, the DVT rates were found to be 13% in the UFH group and 29% in the placebo group (relative risk reduction being 0.65). Rates of bleeding, PE, and mortality were not reported. Subsequent demonstration that leg scanning is not a reliable diagnostic test for DVT limits the utility of this study. In a multicenter trial, Fraisse and colleagues randomized 223 patients mechanically ventilated for chronic obstructive pulmonary disease to a once-daily administration of the low-molecular-weight heparin (LMWH), nadroparin, or placebo. Patients had weekly ultrasonography, and venography was recommended at 21 days or earlier if results from ultrasonography were positive or nondiagnostic. The proportion of patients developing DVT was 16% in the nadroparin group and 28% in the placebo group (relative risk reduction being 0.45). There was a trend toward increased overall bleeding (25 vs 18 patients, P = .18) and major hemorrhage (6 vs 3 patients, P = .28) in the nadroparin group. PE was not systematically evaluated; 8 patients in each group died ( P = .72), but autopsies were not performed.
In summary, the beneficial effect of thromboprophylaxis on DVT in medical-surgical patients has been documented. However, whether thromboprophylaxis decreases PE rates alone has not been directly determined in randomized trials because of the small sample sizes of the available trials and because only few patients were diagnosed with PE.
Diagnosing PE
The reference standard for the diagnosis of PE is contrast pulmonary angiography. However, contrast pulmonary angiography is rarely performed in the ICU because of technical difficulty, cost, potential for toxicity, and the difficulty of transporting critically ill patients to the radiology department. Computed tomographic pulmonary angiography (CTPA) has replaced direct pulmonary angiography because of its ease of use and widespread availability; however, CTPA is expensive and is associated with the same transport risk and risk of contrast-induced nephropathy. CTPA has a sensitivity of 83% and a specificity of 95% in hospitalized patients. Ventilation-perfusion lung scanning, although widely used outside the ICU, is not too useful during critical illness, because ventilation cannot be easily performed in mechanically ventilated patients, and a prevalent pulmonary pathologic condition in the ICU often creates indeterminate results. Other tests such as transthoracic echocardiography showing features such as dilatation of the right side of the heart are suggestive but not diagnostic of PE.
Because pulmonary angiography and CTPA are performed in only a small proportion of critically ill patients who are deemed stable for transport and unlikely to develop contrast nephropathy and because lung scans are rarely interpretable, a large proportion of critically ill patients who have clinical signs of PE do not undergo objective testing. Such patients are either treated empirically or diagnosis is considered but not pursued because of the lack of a safe, available, and validated evaluative regimen; that is, in addition to the low pretest probability of PE in the ICU setting, there is a low rate of objective testing in practice.
As demonstrated in trauma patients, the diagnosis of PE is very challenging during critical illness. In a trauma ICU, 48 of 972 patients with a 10% or greater sudden decrease in oxygen saturation (Sa o 2 ) but no changes in static lung compliance underwent pulmonary arteriography (PA) ; 21of 48 (44%) had PE on a PA test and either a clear chest radiograph or no change in previous pulmonary pathology. Investigators suggested that trauma patients who have more than 10% decrease in Sa o 2 without changes in lung compliance or chest radiograph findings should undergo PA—an approach, which yields a sensitivity, specificity, and predictive value of 100%, 99.9%, and 95%, respectively, for the diagnosis of clinically significant PE. However, performing PA in all medical-surgical critically ill patients who have greater than 10% decrease in Sa o 2 and no change in lung compliance or chest radiograph findings is premature for the medical-surgical ICU population.
In clinical trials, because of the difficulty in interpreting some test results, central adjudication is often used to categorize PE outcomes. For blinded and unblinded trials in which outcome ascertainment bias is minimal, and when objective outcomes are measured, adjudication is generally unnecessary. However, outcome adjudication for events such as PE is desirable because the outcome is subject to ascertainment bias; clinical definitions vary, creating poor reproducibility; and outcome assessment requires specialized knowledge, making misclassification common. In such circumstances, PE is sometimes classified as follows: (1) definite PE, (2) probable PE, (3) possible PE, and (4) no PE. Common definitions are definite PE: clearly positive test result without reference to a clinical suspicion or pretest probability, probable PE: moderate to high pretest probability (high clinical suspicion) plus no test or a nondiagnostic test, possible PE: low pretest probability (low clinical suspicion) plus a nondiagnostic test, and no PE: clearly negative test result.
In summary, PE is underdiagnosed during critical illness because of the nonspecific nature of the presentation, low rates of testing, and challenging test-result interpretation. For clinical trial purposes, patients with suspected PE often undergo adjudication by a blinded review committee. Typically, PE is diagnosed by an intraluminal filling defect on either chest computed tomography (CT) or direct pulmonary angiogram. For those critically ill patients who are not mechanically ventilated, segmental or greater perfusion defects on lung scans, with a normal chest radiograph in the area of the defect are also diagnosed as having PE in the setting of a compatible clinical history.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree