Pulmonary Disease
1 Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA
2 The Commonwealth Medical College, Scranton, PA
3 The Warren Alpert Medical School of Brown University, Providence, RI
Chronic Obstructive Pulmonary Disease (Chapter 9: Case 1)
Between 25 and 40 percent of patients with advanced chronic obstructive pulmonary disease (COPD) have some degree of nutritional depletion. Weight loss, with reductions in fat reserves and muscle mass, occurs in 30 percent of patients with COPD. Patients who lose 15 percent or more of their weight within a year are at risk for malnutrition, which is associated with a higher mortality even after adjusting for age, smoking habits, baseline BMI, and lung function. Mean survival of COPD patients with a low BMI is considerably shorter than those who are not underweight.
However, even patients at normal body weight may be undernourished. The prevalence of malnutrition may be underestimated when BMI alone is used for assessment. Fat-free mass (FFM) index is a better marker of lean body mass compared to BMI because it is associated with prognostic indices such as six-minute walk distance, dyspnea, percentage of predicted FEV1 and FEV1/FVC ratio, airway obstruction, lung hyperinflation, and total lung capacity. Depletion of FFM with preservation of body weight occurs in 11 to 25 percent of COPD patients and is associated with impaired peripheral muscle strength. More severe COPD is associated with an increased risk of malnutrition, as weight loss leads to a reduction in the mass of the respiratory muscles and the diaphragm.
Patients with COPD benefit from nutritional assessment because the consequences of malnutrition include adverse effects on respiratory muscle mass and function that result in decreased respiratory muscle strength and exercise capacity. Furthermore, because malnutrition is also associated with decreased cell-mediated immunity, altered immunoglobulin production, and impaired cellular resistance of the tracheobronchial mucosa to bacterial infection, these patients are at increased risk for respiratory infections, especially pneumonia and bronchitis. Patients with advanced COPD are also at risk for osteoporosis. Low BMI and decreased weight-bearing exercise capacity are independent predictors of osteoporosis.
Interestingly, an imbalance in oxidative status in the setting of malnutrition and low body weight may play a vital role in the pathogenesis and severity of COPD. The imbalances between the formation of reactive oxygen species and antioxidant capacity can cause cell damage, mucous hypersecretion, antiprotease inactivation, and increased pulmonary inflammation. Dietary intake of antioxidant nutrients, including vitamin C, vitamin E, β-carotene, and selenium, has been positively associated with increased lung function. In fact, studies suggest that foods high in antioxidants, including tea, fruits and vegetables and whole grains, may have protective or ameliorative effects on the development of COPD.
Mechanisms of Weight Loss in Patients with COPD
The causes of weight loss in patients with advanced COPD are multiple and still not fully understood. However, they can be separated into processes or conditions that result in poor nutritional intake, altered protein metabolism and a hypermetabolic state. Mechanisms of weight loss in patients with chronic obstructive pulmonary disease are shown in Figure 9-1.
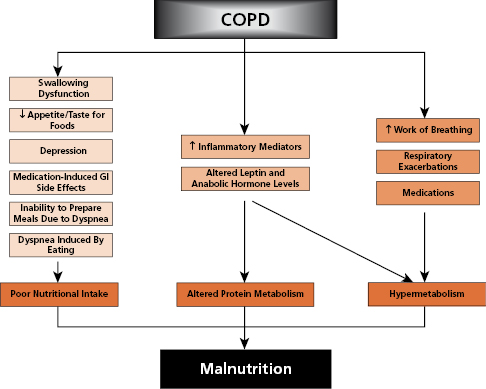
Source: Horace M. DeLisser, MD. 2014. Used with permission.
Poor Nutritional Intake
Factors that may cause poor dietary intake in patients with COPD include:
- Reduced appetite by up to 45 percent in cachectic patients.
- Chronic sputum production and frequent coughing, which may alter the desire for and taste of food and may interfere with swallowing (deglutition).
- Severe dyspnea and fatigue, which may result in an inability to prepare adequate meals.
- Depression from the illness, which may result in anorexia.
- Hyperinflation of the lungs, which causes flattening of the diaphragm and pressure on the abdominal cavity during eating, leading to early satiety and problems with swallowing.
- Oxyhemoglobin desaturation during eating, which results in increased dyspnea.
- Side effects of medications such as nausea, vomiting, diarrhea, dysgeusia, dry mouth, and gastric irritation. Medications may also increase the need for protein, calcium, vitamin A, and folic acid or result in altered serum levels of potassium, magnesium, vitamins, or cholesterol.
- Depression from the illness, which may result in anorexia.
Hypermetabolism
Several causes of hypermetabolism result in increased energy requirements in patients with COPD. These include:
Increased Work of Breathing In patients with normal lung function, breathing expends 36 to 72 calories per day. Patients with COPD may have up to a tenfold increase in their daily energy expenditure from breathing. Both the increased resistive load and the reduced respiratory muscle efficiency experienced by these patients contribute to this increased daily energy expenditure from breathing. This increased work of breathing results in an increased daily energy requirement. Patients will lose weight if they do not ingest additional calories to meet these increased needs. Alternatively, patients may reduce their activity in an effort to conserve energy.
Frequent, Recurrent Respiratory Infections Depending on the severity of the illness, respiratory infections may increase metabolic rate and, therefore, contribute to weight loss.
Miscellaneous Processes Other potential causes for hypermetabolism include disease-induced inflammatory mediators and the use of corticosteroids, β2-agonists, and/or theophylline.
Altered Protein Metabolism Recent studies suggest that elevated levels of the cytokines, especially TNF-α, contribute to weight loss, skeletal muscle loss, and increased resting energy requirement in patients with COPD. Low levels of serum leptin and testosterone have been found in patients with COPD, which are believed to cause increased protein catabolism. Increased levels of growth hormone in cachectic COPD patients may indicate growth hormone resistance. Reductions in phosphocreatine decrease lactate anaerobic metabolism, resulting in early onset lactic acidosis and exercise intolerance. Several studies suggest that the metabolism of the amino acid leucine is abnormal in patients with severe COPD. In summary, protein degradation may increase when inflammatory mediators and stress hormones overwhelm processes that decrease protein turnover.
Medical Nutrition Therapy for COPD
Patients with COPD have difficulty meeting caloric requirements and frequently lose weight. Based on survival statistics, the goal for underweight patients is weight gain and the goal for overweight patients is weight maintenance. It is safe to assume that patients who are not ingesting their caloric requirements and present with weight loss may also suffer from vitamin and mineral deficiencies. Certain electrolytes (calcium, magnesium, potassium and phosphorus) are especially important because depletion may contribute to the impairment of respiratory muscle function. When severely undernourished COPD patients are rapidly re-fed with glucose infusions, careful attention must be paid to these electrolytes to avoid refeeding syndrome (Chapter 4: Case 2).
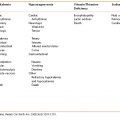
The goals of medical nutrition therapy for COPD patients are shown in Table 9-1.
Table 9-1 Medical Nutrition Therapy for Patients With COPD
Source: Jennifer Williams, MS, RD, CNSD. 2014. Used with permission.
|
Emerging areas of research include the use of anabolic steroids, growth hormones, and appetite stimulants for anabolism. The peptide ghrelin stimulates growth hormone secretion, food intake, and weight gain. Though appetite stimulants often result in an increase in adiposity, ghrelin improves body composition by decreasing muscle wasting via inhibition of production of anorectic proinflammatory cytokines. Appetite stimulants may cause hyperglycemia, which may be exacerbated by steroid medication.
Mechanical Ventilation
Rationale for Nutrition Support
Patients with respiratory failure requiring mechanical ventilation are unable to ingest food through the mouth because of the endotracheal or nasotracheal tube (unless the patient has a tracheostomy). Because many patients require mechanical ventilation for prolonged periods, nutrition support is necessary to prevent malnutrition.
Malnutrition associated with critical illness impairs cell-mediated immunity, alters immunoglobulin production, and impairs cellular resistance to infection. Therefore, patients who have not been fed for 7 to 10 days are at increased risk of infection. In addition, malnutrition causes difficulty in weaning a patient from the ventilator, presumably due to respiratory muscle weakness. Conversely, ventilated patients with pre-existing malnutrition who are fed have improved respiratory muscle strength and function, which may facilitate weaning from the ventilator.
Immune-Modulating Enteral Feeding Formulas
Investigations have demonstrated that early enteral nutrition leads to decreased infections, reduced hospital length-of-stay, and even a reduction in mortality. It has also been noted that a high content of omega-6 polyunsaturated fatty acids (PUFAs) is unfavorable because of the potential to induce a pro-inflammatory state. This has led to the development of emulsions in which part of the omega-6 fatty acid component is replaced by less bioactive fatty acids, such as omega-3 derived from fish oils. Intravenous fish oil has been shown to blunt the physiological response to endotoxin in healthy subjects. Due to increased interest, immune modulating formulas were created and mainly include enteral formulas containing glutamine, arginine, omega-3 fish oils and anti-oxidants. Initially, omega-3 fish oils had been found to improve outcomes in patients with acute lung injury (ALI) or acute respiratory distress syndrome (ARDS). Because of these findings, a grade A (highest) recommendation was provided by the American Society for Parenteral and Enteral Nutrition/Society of Critical Care Medicine guidelines for the use of omega-3 fatty acids in patients with ARDS. Later trials aimed at defining the potential benefits of omega-3s and antioxidants for patients with ALI/ARDS showed less positive results. Further clarification is needed regarding the selection and dosage of antioxidant components for immunonutrition.
There has also been interest in the use of enteral nutrition support, enriched with glutamine. Glutamine enhances the immunological barrier in the GI tract via its trophism of enterocytes and colonocytes and serves as a substrate for glutathione, an antioxidant. Evidence of its potential benefit have come from studies showing a reduction in length of stays, both in the intensive care unit (ICU) and in the hospital, when patients received enteral nutrition support containing glutamine compared to those patients fed an enteral diet without glutamine.
Feeding Options
Most patients who require mechanical ventilation for more than several days should receive enteral nutrition via a naso-enteral feeding tube as long as the GI tract is functioning (Chapter 12). Parenteral nutrition should be reserved for patients who are severely undernourished and/or do not have a functioning gut such as those with a bowel obstruction or an ileus (Chapter 13).
Minimizing Effects of Nutrition Support on CO2 Production
The caloric and nutrient composition of the diet has a profound effect on gas exchange, especially CO2 production. The respiratory quotient (RQ) is expressed as the ratio of CO2 produced to oxygen consumed.
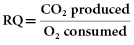
The RQ of carbohydrate is 1.0, while the RQ of fat is 0.7 and of a mixed meal is 0.83. Thus, CO2 production is greater during carbohydrate metabolism than during fat metabolism. A diet high in carbohydrate therefore requires increased ventilation to eliminate the excess CO2 and may complicate weaning from the ventilator. Consequently, high-fat, low-carbohydrate enteral feeding products have been formulated and recommended for feeding mechanically ventilated patients with severe COPD. These have not been proven effective, however, probably because excess CO2 production associated with mixed or high-carbohydrate diets is not clinically relevant unless caloric requirements are exceeded. Thus, it is essential to avoid overfeeding these patients because this can result in excessive CO2 production, increased RQ, and difficulty in weaning from the ventilator. If indirect calorimetry is available to determine caloric expenditure, this should be recommended; otherwise 100 to 120 percent of predicted caloric expenditure should be used.
Cystic Fibrosis (Chapter 9: Case 3)
Cystic fibrosis (CF), a life-threatening genetic disorder, manifesting in child1ren and young adults, presents with profuse, abnormally thick exocrine gland secretions. These excessive secretions may obstruct pancreatic and bile ducts, intestines, and bronchioles, resulting in a variety of clinical problems, with chronic lung disease and pancreatic insufficiency being the most common. There is a clear association between worsening lung disease and malnutrition, but the degree of malnutrition seen in these patients varies considerably. Deficiencies of specific micronutrients may progress to clinically evident symptoms and signs if not recognized and treated. In addition, deficiencies of calories, protein, essential fatty acids, fat-soluble vitamins (A, K, E, and D), beta-carotene, zinc, iron, and sodium have been reported for these patients. Bone disease, such as osteoporosis, is also being increasingly recognized.
Causes of Weight Loss and Malnutrition
The causes of weight loss and malnutrition in patients with CF are multifactorial. These include maldigestion and/or malabsorption (due to pancreatic insufficiency), inadequate oral caloric intake, increased caloric and nutrient needs, and the development of CF-related organ system disease, particularly pulmonary disease, liver disease, intestinal obstruction, and CF-related diabetes mellitus (CFRD).
Maldigestion and Malabsorption Most patients (80 to 85 percent) with CF have pancreatic insufficiency and as a result malabsorption of fats, proteins, carbohydrates, vitamins, and minerals, which, if untreated, leads to serious nutritional problems. Pancreatic enzyme supplements are administered with meals and snacks to assist with the absorption of nutrients. The amount and type of enzyme supplements depend on the degree of malabsorption and the fat content of the diet. Steatorrhea is considered a clinical indicator of fat malabsorption (Chapter 7: Case 2).
Increased Nutritional Needs Despite pancreatic enzyme supplementation, the energy and protein needs of CF patients are significantly increased. This is due to loss of nutrients secondary to malabsorption and by higher than normal protein catabolism and energy expenditure due to frequent infections.
Increased Work of Breathing Patients with CF commonly suffer from chronic bronchitis, airway obstruction, and recurrent infections, which increase the work of breathing and result in higher energy expenditure. Muscle wasting and respiratory muscle dysfunction further exacerbates these effects.
Other Factors Gastro-esophageal reflux, abdominal pain, and psychosocial stress may also contribute to low caloric intake. Liver disease with decreased bile salt excretion also worsens malabsorption. CF-related diabetes mellitus with glucosuria also results in increased energy loss. Finally, patients who undergo significant intestinal resection may have decreased intestinal surface area for nutrient absorption.
Medical Nutrition Therapy for Cystic Fibrosis
Patients with CF are typically unable to meet their caloric and protein requirements or maintain their weight due to increased nutrient needs and losses, and inadequate caloric intake. CF is usually diagnosed in infancy or early childhood, and monitoring growth and development in these patients is particularly important. Not uncommonly, CF patients remain at or fall below the fifth percentile in both weight-for-age and height-for-age on pediatric growth charts. The goals of nutrition therapy for CF patients:
- Routine nutrition assessment, which includes height, weight, BMI, percent weight change, pediatric growth parameters, dietary history, physical examination, and evaluation of laboratory values.
- Dietary counseling to provide adequate intake of calories, protein, vitamins, and minerals. This will include education about high-calorie, balanced meals with added salt, nutrient-dense snacks two to three times daily, and nutritional supplements.
- Adequate pancreatic enzyme replacement therapy adjusted to avoid malabsorption.
- Adequate vitamin and mineral supplements according to the CF Foundation Guidelines.
If patients continue to experience weight loss and fall below 85 percent of their ideal body weight, additional nutrition support may be necessary. Both enteral (using nasogastric or gastrostomy tubes) and parenteral feedings may be recommended, as clinically indicated.
Obstructive Sleep Apnea (Chapter 9: Case 2)
Obstructive sleep apnea (OSA) is defined as recurrent episodes of apnea during sleep caused by occlusion of the upper airway. Obesity is the primary risk factor for OSA and is present in up to two-thirds of all patients with OSA. OSA may be caused by an increased amount of fat surrounding the structures of the upper airway. Although not all obese patients have OSA, and occasional non-obese patients may have it, it is clear that weight loss in obese patients with OSA improves signs and symptoms. Symptoms of sleep apnea, such as snoring and excessive daytime sleepiness, should always be ascertained as part of the medical history in obese patients.
Two groups of inflammatory proteins are produced and released by adipose tissue: cytokines, such as TNF-α and adipokines, such as leptin. These cytokines may play a role in the development of insulin resistance and increased oxidative stress in obese patients.
Studies have suggested a role for the fat cell protein, leptin, in the pathogenesis of respiratory dysfunction in OSA. In fact, mutation in the leptin or leptin receptor gene has been found in some obese human subjects. Other investigations suggest that patients with OSA also have lower plasma levels of orexin, a neuropeptide produced in the lateral hypothalamus, that increases appetite and alertness. It has been proposed that lower levels of orexin may result in decreased levels of alertness and may play a role in the pathogenesis of OSA.
In addition to weight loss, patients with OSA are most commonly treated with continuous positive airway pressure (CPAP) therapy. A CPAP machine is approximately the size of a toaster, and is connected to tubing that ends with a mask that must be worn snugly over the face. The machine blows air into the throat and splints the airway open during sleep. CPAP eliminates apneas and snoring.
Causes of Weight Gain and Obesity
Fatigue due to chronic sleep disruption, a common symptom of patients with OSA, may influence patients’ eating behaviors. Often too tired and lacking in motivation to exercise, they tend to lead sedentary lifestyles. In addition, many patients with OSA report falling asleep often after eating, which further decreases their energy expenditure. Certain overweight patients with OSA may also be prone to binge eating as a result of depression about their illness and/or body image. Whatever the exact causes, a combination of decreased physical activity and increased caloric consumption contributes to weight gain in these patients.
Medical Nutrition Therapy for Obstructive Sleep Apnea
Weight Loss Because obesity contributes to the pathogenesis of OSA, weight loss is of primary importance in obese patients with OSA. Weight loss, even as small as 5 to 10 percent body weight, can dramatically improve breathing and sleep patterns. Patients would benefit from a referral to a registered dietitian for either individual or group nutritional counseling.
Increasing Activity Once patients begin to feel better and have more energy, they should be encouraged to begin a low-intensity exercise program, such as walking 15 minutes once or twice a day.
Lung Transplantation
Lung transplantation has become a viable alternative for some patients with severe pulmonary disease, including COPD, cystic fibrosis, and pulmonary hypertension. The nutritional implications for lung transplantation patients vary depending on whether they are waiting for or have received a transplant, and whether they are breathing spontaneously or mechanically ventilated following surgery. The following recommendations are listed accordingly.
Nutrition Assessment Prior to Lung Transplantation
Routine nutrition assessment prior to lung transplantation entails the following steps:
- Assess nutritional status using BMI and the patient’s weight history, and body composition measurement if equipment is available.
- Assess albumin as a predictor of mortality. If protein status is depleted, supplement the diet with high-protein milkshakes and snacks.
- Assess serum lipid levels.
- Monitor the patient’s satiety level and gastrointestinal symptoms, such as bloating and gas, which could interfere with adequate dietary intake.
- Assess bone density with a DEXA scan pre- and post-lung transplantation.
Medical Nutrition Therapy Post-Lung Transplantation
Several of the drugs used for immunosuppression after lung transplantation have an impact on nutrition. Cyclosporine can cause hyperkalemia, and may also elevate serum cholesterol and triglyceride levels. These effects may require reducing dietary potassium, saturated fat, and cholesterol intake. Tacrolimus, often substituted for cyclosporine, causes hyperglycemia. The antimetabolite azathioprine causes nausea, vomiting, and diarrhea. The similarly acting mycophenolate mofetil may produce diarrhea and dyspepsia. These problems may interfere with the provision of adequate intake and must be addressed.
Corticosteroids (e.g., prednisone) can cause hyperglycemia and increased appetite, which often leads to weight gain and potentially obesity. Patients taking corticosteroids may also experience fluid retention and osteoporosis.
Medical nutrition therapy immediately following lung transplantation is shown in Table 9-2.
Table 9-2 Medical Nutrition Therapy Following Lung Transplant
Source: Jennifer Williams, MS, RD, CNSD. 2014. Used with permission.
|
Case 1 Chronic Obstructive Pulmonary Disease
1 Perelman School of Medicine at the University of Pennsylvania, Philadelphia, PA
2 The Commonwealth Medical College, Scranton, PA
3 The Warren Alpert Medical School of Brown University, Providence, RI
4 Jefferson Medical College, Philadelphia, PA
PD is a 53-year-old Caucasian woman diagnosed with chronic obstructive pulmonary disease (COPD) 8 years ago, who visits her physician complaining of shortness of breath (dyspnea). This has worsened progressively over the last 3 days since she caught a cold from her grandchildren. She explains that her level of dyspnea increases when she is sick or under increased stress, high humidity, extremely cold temperatures, or after a large meal. Currently, PD has two-pillow orthopnea and bilateral lower extremity edema. She reports an unintentional weight loss of 31 pounds (14 kg) within the last year. Pulmonary function tests from last year confirmed severe COPD with a forced expiratory volume (FEV1) of 36 percent predicted, a forced vital capacity (FVC) of 44 percent predicted, and a ratio of FEV1 to FVC of 39 percent. A recent chest X-ray revealed hyperinflation of lung fields, with diminished lung markings in the upper lung fields.
Past Medical History
PD has been treated for hypertension for 12 years and for hypercholesterolemia for the past 2 years. She has no previous history of diabetes mellitus, thyroid disease, or liver disease.
Medications
PD is currently taking verapamil, furosemide, potassium chloride, atorvastatin, prednisone, tiotropium bromide, alendronate, and albuterol. She does not take any vitamin/mineral or herbal supplements. PD has no known food allergies.
Family History
PD’s mother died at age 70 of a heart attack. Her father also died of a heart attack at age 73.
Social History
PD lives with her husband in a two-story home. They have 4 children and 14 grandchildren. PD worked in a local department store as a salesperson until last year, when she retired because of her illness. She formerly attended church regularly with her husband, but lately has been too tired. Her husband has also recently taken over the food shopping. She reports the following substance use:
- Alcohol intake: None
- Tobacco: 1½ packs per day for 30 years; quit 5 years ago
- Caffeine: One cup of coffee/day
Diet History
PD is on a low-fat, low-cholesterol, low-salt diet for elevated cholesterol and hypertension. PD provided the following 24-hour dietary recall that reflects her typical daily intake. She does not add salt to her food or use salt in cooking.
PD’s 24-Hour Dietary Recall
| Breakfast (home) | |
|---|---|
| Cream of wheat | 1.5 cup cooked |
| White toast | 1 slice |
| Jelly | 2 Tbsp. |
| Coffee | 1 cup |
| 1% milk | 4 ounces (120 mL) |
| Lunch (home) | |
| Low-fat yogurt | 1 cup |
| Apple juice | 6 ounces (180 mL) |
| Dinner (home) | |
| Baked chicken breast | 4 ounces (114 g) |
| Baked potato | 1 medium |
| Cooked carrots | ½cup |
| Diet margarine | 1 Tbsp. |
| Water | 1 glass |
| Snack (home) | |
| Banana | 1 medium |
- Total calories: 1262 kcal
- Protein: 63 g (20% of calories)
- Fat: 21 g (15% of calories)
- Saturated fat: 7.0 g (5% of calories)
- Monounsaturated fat: 5.0 g (4% of calories)
- Cholesterol: 112 mg
- Carbohydrate: 209 g (66% of calories)
- Fiber: 11 g
- Sodium: 1036 mg
Review of Systems
- General: Weakness, fatigue, and weight loss
- Mouth: Wears dentures (top and bottom; loose fitting)
- GI: Poor appetite; no nausea, vomiting, or diarrhea; daily bowel movements
- Extremities: No joint pain; has difficulty walking without a walker
Physical Examination
Vitals Signs
- Temperature: 97 °F (36 °C)
- Heart rate: 94 BPM
- Respiration: 20 BPM
- Blood pressure: 150/80 mm Hg
Anthropometric Data
- Height: 5′6″ (168 cm)
- Current weight: 134 lb (61 kg)
- Estimated dry weight: 112 lb (51 kg)
- [Dry weight is estimated by subtracting the weight of the fluid from the current weight. Fluid weight is estimated at 22 pounds (10 kg) since she has 2+ pitting edema on both ankles. Eleven pounds (5 kg) can be used to estimate fluid in patients with ascites and no peripheral edema]
- Usual dry weight: 143 lb (65 kg)
- BMI using estimated dry weight: 18 kg/m2
- Percent weight change using estimated dry weight (over 1 year): 22% decrease [(65 − 51)/65]
Exam
- General: Frail woman in no acute distress
- Skin: Ecchymoses
- HEENT: Normal non-palpable thyroid
- Mouth: Loose-fitting dentures; no sores; symmetrical soft palate and uvula
- Cardiac: Regular rate and rhythm; normal first and second heart sounds; jugular venous distention and hepatojugular reflux noted
- Lung: Increased A–P diameter, decreased breath sounds throughout; diffuse mild expiratory wheezing with a prolonged expiratory phase
- Abdomen: Non-distended, non-tender; no hepatosplenomegaly; normal bowel sounds
- Extremities: 2+ pitting edema on both ankles
- Rectal: Soft, heme-negative brown stool in vault
- Neurologic: Alert; appropriate reactions; good memory; no evidence of sensory loss
Laboratory Data
| Patient’s Values | Normal Values |
|---|---|
| Albumin: 4.3 g/dL | 3.5–5.8 g/dL |
| Hemoglobin: 10.8 g/dL | 12.0–16.0 g/dL |
| Hematocrit: 35% | 36–46% |
| Mean corpuscular volume: 78 fL | 80–100 fL |
| Cholesterol: 265 mg/dL | desirable <200 mg/dL |
| LDL-C: 173 mg/dL | desirable <130 mg/dL |
| HDL-C: 42 mg/dL | desirable >40 mg/dL |
| Triglycerides: 150 mg/dL | desirable <150 mg/dL |
| Arterial blood gases (ABG): | |
| pH: 7.37 | 7.35–7.45 |
| pCO2: 63 mm Hg | 33–45 mm Hg |
| pO2: 60 mm Hg | 80–100 mm Hg |
| HCO3: 35 mEq/L | 24–28 mEq/L |
| SaO2: 90% | 95–100% |
Case Questions
- Does PD’s percent weight change indicate a significant weight loss?
- Estimate PD’s caloric needs using the Mifflin–St. Jeor equation including a stress factor for COPD.
- What factors have contributed to PD’s weight loss?
- Based on PD’s history, what may account for her severe fatigue?
- How does poor nutritional status compromise pulmonary function?
- Discuss the impact of current medications on nutritional status.
- What is the appropriate medical nutrition therapy for PD, including specific recommendations to improve her nutritional and fluid status?
Answers to Questions: Case 1
Part 1: Nutrition Assessment
1. Does PD’s percent weight change indicate a significant weight loss?
Progressive, unintentional weight loss of greater than 5 percent in 1-month or greater than 15 percent of body weight within a 1-year period is considered a severe weight loss, and represents a significant risk for malnutrition. PD had an unintentional weight loss of 22 percent over the past year, and her current BMI is less than 18.5 kg/m2, both considered a risk for malnutrition.
2. Estimate PD’s calorie needs using the Mifflin–St. Jeor equation including an activity factor for COPD.
The Mifflin–St. Jeor equation for women:
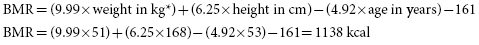
*Use estimated dry weight in this patient with bilateral pitting edema.
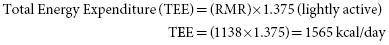
When the TEE is compared to her current intake, which totals 1262, her calorie needs are about 300 greater than her actual intake. This could explain her continued weight loss.
3. What factors have contributed to PD’s weight loss?
Because of reduced lung function, PD requires more energy to breathe. The normal daily intake of calories required to maintain her body weight is insufficient to meet the excessive demands of breathing for COPD patients. Elevated cytokines (e.g., TNF-alpha) and decreased levels of cell-derived protein (e.g., leptin and testosterone) exacerbated by frequent recurrent respiratory infections increase resting energy requirements and promote loss of weight and lean body mass.
PD’s diet history reveals that her calorie intake meets only 80% of her nutritional requirements. Her low calorie intake is due in part to the low-fat, low-cholesterol diet originally prescribed to manage her hypertension and hypercholesterolemia. Patients with pulmonary disease may ingest even fewer calories because they are too tired to prepare food or to eat a meal. Such patients report dyspnea while chewing and swallowing food, preventing them from breathing adequately and, thereby, increasing the amount of desaturation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree