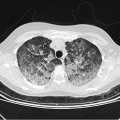
Fig. 10.1
Diagnostic procedures and treatment of neutropenic patients with fever and suspected or proven lung infiltrates. CT computed tomography, BAL bronchoalveolar lavage. Dotted lines indicate exceptions from recommended procedure
Patients with fever of unknown origin not responding to an appropriate first-line therapy after 72–96 h should undergo thorough physical reexamination, imaging, and microbiological diagnostics including thoracic CT scan (see chapter on FUO). When LI are documented, bronchoscopy and BAL should promptly be arranged. BAL samples must be sent immediately to the microbiological laboratory for workup, to be started within 4 h after sampling. Recommended microbiological procedures are listed in Table 10.1.
Table 10.1
Workup of bronchoalveolar lavage samples from febrile neutropenic patients with lung infiltrates [95]
Recommended diagnostic program |
Cytospin preparations for distinguishing intracellular from extracellular pathogens and identifying infiltration by underlying malignancy |
Gram stain |
Giemsa/May-Grünwald-Giemsa stain (assessment of macrophages, ciliated epithelium, leukocytes) |
Calcofluor white or equivalent (assessment of fungi and Pneumocystis jirovecii) |
Direct immunofluorescence test for Pneumocystis jirovecii (confirmatory) |
Direct immunofluorescence test for Legionella spp. |
Ziehl-Neelsen/auramine staining |
Aspergillus antigen (galactomannan sandwich ELISA) |
Quantitative cultures: dilutions of 10−2 and 10−4; culture media: blood, McConkey/Endo, Levinthal/blood (bacterial culture), Legionella BCYE-α or equivalent (Legionella spp.), Löwenstein-Jensen or equivalent (mycobacteria), Sabouraud/Kimmig or equivalent (fungal culture) |
Optional program |
Enrichment culture (brain-heart infusion, dextrose broth) |
Direct immunofluorescence test for Chlamydia pneumoniae |
Culture for Chlamydia pneumoniae |
Legionella PCR |
Shell vial technique and PCR for influenza, parainfluenza, and adenovirus |
Culturing or antigen detection of herpes simplex and varicella zoster virus |
Cytomegalovirus early antigen |
CMV antibody (ELISA, IgG/IgM) |
HSV antibody (ELISA, IgG/IgM) |
VZV antibody (ELISA, IgG/IgM/IgA) |
Respiratory syncytial virus (PCR, ELISA) |
Panfungal/Aspergillus PCR |
Peripheral blood cultures 1 h after bronchoscopy to detect transient bacteremia |
Throat swab to assess oral flora in comparison with BAL |
Pneumocystis jirovecii PCR |
Invasive procedures such as open-lung or fine-needle biopsy should be considered in patients with undetermined LI who urgently require histological identification.
10.4 Antimicrobial Therapy
Considering the dismal prognosis of febrile neutropenic patients with LI not treated promptly with an appropriate antimicrobial regimen, it is recommended to start therapy preemptively on the basis of clinical, imaging, and/or laboratory findings indicative of a particular infection in patients at risk for, but without proof of, this infection (Table 10.2). The type of underlying malignancy or immunosuppression has an instrumental impact on the selection of antimicrobial agents suitable for preemptive therapy.
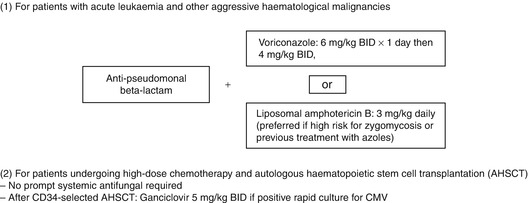
Table 10.2
Preemptive antimicrobial treatment in febrile neutropenic patients with lung infiltrates [37]

10.4.1 Patients with Severe Neutropenia Due to Chemotherapy for Acute Leukemia or Other Aggressive Hematologic Malignancies
In this subgroup of neutropenic patients with LI, broad-spectrum beta-lactam therapy with antipseudomonal activity, as used for empirical treatment of unexplained fever (FUO => see separate chapter), should primarily be combined with a mold-active systemic antifungal, i.e., voriconazole (6 mg/kg every 12 h day 1, 4 mg/kg every 12 h thereafter) or liposomal amphotericin B (3 mg/kg daily) [153]. In patients in whom zygomycosis (mucormycosis) is suspected or who have been pretreated with voriconazole or posaconazole, liposomal amphotericin B is preferred. This high-risk subgroup of patients has a significant benefit from prompt as compared to delayed mold-active antifungal therapy [130]; it has been shown that patients with invasive aspergillosis treated with voriconazole or liposomal amphotericin B had superior response and survival rates when treated early vs. later in the course of the disease [37, 60]. Systemic antifungal treatment should be continued until hematopoietic recovery and regression of clinical and radiological signs of infection.
The empirical addition of an aminoglycoside or 5-flucytosine is not recommended due to a lack of benefit [116]. In patients who had not taken routine anti-Pneumocystis prophylaxis, who have a thoracic CT scan suggesting PcP, and who have a rapid and otherwise unexplained rise of serum lactate dehydrogenase, prompt start of high-dose co-trimoxazole therapy should be considered before bronchoscopy and BAL. In case of PcP, BAL will remain positive for this pathogen over several days despite appropriate antimicrobial therapy.
Except from patients with severe cellular immunosuppression, antiviral agents such as ganciclovir are not recommended for early preemptive therapy in febrile neutropenic patients with LI. In general, glycopeptides or macrolide antibiotics without a specific pathogen isolated from clinically significant samples should not be used as well.
10.4.2 Other Subgroups of Febrile Patients with Hematologic Malignancies
In patients undergoing high-dose chemotherapy and autologous hematopoietic stem cell transplantation (AHSCT) with febrile neutropenia and LI of unknown origin, whose conditioning regimen included total body irradiation or who have received a CD34-selected autograft [71], bronchoscopy with BAL should be performed to check for CMV disease [53]. A positive rapid culture or “immediate early antigen” should prompt ganciclovir treatment (5 mg/kg every 12 h). Foscarnet has not been investigated in this setting, as has not been the serial blood PCR or pp65 antigen monitoring. Since patients after AHSCT have a very low risk of fungal pneumonia [112, 118, 121], antifungal therapy should not be given preemptively.
10.5 Antimicrobial Treatment in Patients with Documented Pathogens
The interpretation of microbiological findings in neutropenic patients with LI is difficult. Isolates typically origin from blood cultures or BAL samples. They may represent nonpathogenic contaminants, colonizers, co-pathogens, or microorganisms causing a separate infection. If etiologically significant pathogens are detected, particularly multiresistant bacteria, immediate modification of antimicrobial treatment to avoid fatal outcome due to delayed effective therapy is recommended [75].
The following findings indicate pathogens causative for lung infiltrates:
Pneumocystis jirovecii, gram-negative aerobic pathogens, pneumococci, Mycobacterium tuberculosis, or Aspergillus spp. or Aspergillus galactomannan or zygomycetes obtained from bronchoalveolar lavage or sputum samples; positive rapid culture for CMV and detection of CMV “immediate early antigen”
Isolation of pneumococci, alpha-hemolytic streptococci, or gram-negative aerobic pathogens from blood culture
Any detection of pathogens in biopsy material
Positive Legionella antigen in urine
Positive (threshold 0.5) Aspergillus galactomannan in blood samples or BAL
The following findings do not represent pathogens causative for lung infiltrates:
Isolation of enterococci from blood culture, swabs, sputum, or BAL
Coagulase-negative staphylococci or Corynebacterium spp. obtained from any sample
Isolation of Candida spp. from swabs, saliva, sputum, or tracheal aspirates
Findings from surveillance cultures, feces, and urine cultures
Potentially relevant findings are common respiratory viruses; isolation of Staphylococcus aureus, Legionella spp., or atypical mycobacteria in respiratory secretions; and positive CMV- or Pneumocystis-PCR from BAL.
In patients with a documented P. aeruginosa pneumonia, the primary combination antibacterial therapy including an antipseudomonal beta-lactam plus preferably an aminoglycoside or (if an aminoglycoside is contraindicated) ciprofloxacin is recommended [54, 114]. Depending on their in vitro susceptibility pattern, multiresistant gram-negative aerobes such as extended-spectrum beta-lactamase (ESBL)-producing E. coli, Enterobacter spp. or Klebsiella spp., as well as Acinetobacter spp. or P. aeruginosa require antimicrobial treatment combinations selected appropriately according to this pattern. Pharmacokinetic aspects (penetration to lung tissue, possible inactivation by surfactant) must always be included in this selection. In individual patients with pneumonia caused by multiresistant gram-negative pathogens, aerosolized colistin has been successfully used as a part of the antimicrobial strategy [100]. Stenotrophomonas maltophilia rarely causes pneumonia, while it is more frequently isolated from respiratory secretions representing selection of opportunistic microorganisms under broad-spectrum antibacterial treatment. In patients with suspected or documented S. maltophilia pneumonia, early antimicrobial intervention with high-dose trimethoprim-sulfamethoxazole (15–20 mg/kg/day of trimethoprim) is mandatory [2, 145]. It should be kept in mind that in vitro susceptibility may not predict clinical efficacy of antimicrobial agents in S. maltophilia infections [30].
Pneumonias caused by methicillin-resistant Staphylococcus aureus (MRSA) should preferably be treated with vancomycin, if no serious renal insufficiency is present. Linezolid is a valid alternative for first-line treatment [158]; however, the risk of severe thrombocytopenia or even pancytopenia associated with linezolid must be taken into consideration [51]. Daptomycin should not be used for treatment of pneumonia, because it is inactivated by surfactant [134].
CMV pneumonia typically affects patients who have undergone allogeneic stem cell transplantation. First-choice antiviral treatment options are foscarnet and ganciclovir. Foscarnet may be preferred because of its lack of myelosuppression, the latter being a serious adverse effect of ganciclovir [102]. On the other hand, reversible nephrotoxicity is one of the typical side effects of foscarnet [152].
10.6 Treatment of Documented Fungal Pneumonia
Detailed recommendations for treatment of documented fungal pneumonia are provided in evidence-based guidelines [18, 83, 153]. Intravenous voriconazole and liposomal amphotericin B are recommended first-line choices for treatment of IPA. For zygomycosis (mucormycosis), liposomal amphotericin B is preferred. In patients with worsening LI and gas exchange within the first week of treatment, failure of antifungal therapy should only be considered if new LI emerge on control CT scans. At the same time, other causes such as a second infection, immune reconstitution syndrome, infiltrates caused by the underlying malignancy, toxicity from cancer treatment, and yet insufficient duration of antifungal treatment should be ruled out [97]. Combination antifungal first-line treatment in patients with invasive mold infections is controversial. A prospective clinical study comparing voriconazole alone with the combination of voriconazole with anidulafungin has not convincingly shown benefit from the combination in patients with proven and probable aspergillosis [93]. For treatment of zygomycosis (mucormycosis), a combination of liposomal amphotericin B and an echinocandin may be promising [139]; however, randomized studies on this subject have not been conducted. A combination of liposomal amphotericin B and the iron chelator deferasirox for the treatment of mucormycoses has shown inferior clinical results for the combination as compared to the antifungal agent alone [140].
10.7 Treatment of Documented Pneumocystis Pneumonia (PcP)
Patients with proven PcP should be treated with trimethoprim-sulfamethoxazole (TMP/SMX; co-trimoxazole) at a dosage of TMP 15–20 mg/kg plus SMX 75–100 mg/kg daily, divided into three to four doses and continued for 2–3 weeks. Nonresponders after 14 days of treatment should be evaluated for a secondary infection by repeated bronchoscopy. In individual patients with persistent PcP, dihydropteroate synthase gene mutation may be taken into consideration [105]. In case of confirmed sulfa resistance or TMP/SMX intolerance, atovaquone oral suspension (750 mg three times daily), pentamidine inhalation (600 mg daily), intravenous pentamidine (4 mg/kg daily), and clindamycin (600 mg three times daily) plus primaquine (30 mg daily) may represent treatment alternatives [146], with clindamycin + primaquine presumably being the most effective option [137]. Subsequently, patients should be given secondary prophylaxis using oral TMP/SMX at a daily dosage of 160/800 mg given on 2–3 days per week or with monthly pentamidine inhalation at a dose of 300 mg. In patients with respiratory failure due to PcP, systemic corticosteroids may be beneficial, but clinical data from this setting are rare [43, 113].
10.8 Intensive Care Medicine
Analysis of European data on the outcome of cancer patients requiring intensive care has shown an overall mortality rate of 27 %, which is not significantly different from non-cancer patients treated in the intensive care unit [144]. Neutropenic patients with respiratory failure due to LI may have a favorable outcome under appropriate intensive care including mechanical ventilation [35, 96, 110]. Even if respiratory failure is due to invasive pulmonary aspergillosis, survival can be achieved in 33 % of patients [22]. It is therefore not justified to reject cancer patients from intensive care because of their underlying malignancy [131].
References
1.
Ahmed S, Siddiqui AK, Rossoff L, Sison CP, Rai KR. Pulmonary complications in chronic lymphocytic leukemia. Cancer. 2003;98:1912–7.PubMed
2.
Aisenberg G, Rolston KV, Dickey BF, Kontoyiannis DP, Raad II, Safdar A. Stenotrophomonas maltophilia pneumonia in cancer patients without traditional risk factors for infection, 1997–2004. Eur J Clin Microbiol Infect Dis. 2007;26:13–20.PubMed
3.
Akova M, Paesmans M, Calandra T, Viscoli C. A European Organization for Research and Treatment of Cancer-International Antimicrobial Therapy Group study of secondary infections in febrile, neutropenic patients with cancer. Clin Infect Dis. 2005;40:239–45.PubMed
4.
Al Ameri A, Koller C, Kantarjian H, et al. Acute pulmonary failure during remission induction chemotherapy in adults with acute myeloid leukemia or high-risk myelodysplastic syndrome. Cancer. 2010;116:93–7.PubMed
5.
Alanio A, Desoubeaux G, Sarfati C, et al. Real-time PCR assay-based strategy for differentiation between active Pneumocystis jirovecii pneumonia and colonization in immunocompromised patients. Clin Microbiol Infect. 2011;17:1531–7.PubMed
6.
Armenian SH, La Via WV, Siegel SE, Mascarenhas L. Evaluation of persistent pulmonary infiltrates in pediatric oncology patients. Pediatr Blood Cancer. 2007;48:165–72.PubMed
7.
Aubry A, Porcher R, Bottero J, et al. Occurrence and kinetics of false-positive Aspergillus galactomannan test results following treatment with beta-lactam antibiotics in patients with hematological disorders. J Clin Microbiol. 2006;44:389–94.PubMedCentralPubMed
8.
Azoulay E, Bergeron A, Chevret S, Bele N, Schlemmer B, Menotti J. Polymerase chain reaction for diagnosing pneumocystis pneumonia in non-HIV immunocompromised patients with pulmonary infiltrates. Chest. 2009;135:655–61.PubMed
9.
Azoulay E, Darmon M, Delclaux C, et al. Deterioration of previous acute lung injury during neutropenia recovery. Crit Care Med. 2002;30:781–6.PubMed
10.
Azoulay E, Mokart D, Lambert J, et al. Diagnostic strategy for hematology and oncology patients with acute respiratory failure: randomized controlled trial. Am J Respir Crit Care Med. 2010;182:1038–46.PubMed
11.
Azoulay E, Mokart D, Rabbat A, et al. Diagnostic bronchoscopy in hematology and oncology patients with acute respiratory failure. Crit Care Med. 2008;36:100–7.PubMed
12.
Azoulay E, Thiery G, Chevret S, et al. The prognosis of acute respiratory failure in critically ill cancer patients. Medicine. 2004;83:360–70.PubMed
13.
Becker MJ, Lugtenburg EJ, Cornelissen JJ, et al. Galactomannan detection in computerized tomography-based broncho-alveolar lavage fluid and serum in haematological patients at risk for invasive pulmonary aspergillosis. Br J Haematol. 2003;121:448–57.PubMed
14.
Bodey G, Bueltmann B, Duguid W, et al. Fungal infections in cancer patients: an international autopsy survey. Eur J Clin Microbiol Infect Dis. 1992;11:99–109.PubMed
15.
Boeckh M. Complications, diagnosis, management, and prevention of CMV infections: current and future. Hematol Am Soc Hematol Educ Program. 2011;2011:305–9.
16.
Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–67.PubMedCentralPubMed
17.
Boersma WG, Erjavec Z, van der Werf TS, et al. Bronchoscopic diagnosis of pulmonary infiltrates in granulocytopenic patients with hematologic malignancies: BAL versus PSB and PBAL. Respir Med. 2007;101:317–25.PubMed
18.
Böhme A, Ruhnke M, Buchheidt D, et al. Treatment of invasive fungal infections in cancer patients. Ann Hematol. 2009;88:97–110.PubMed
19.
Boonsarngsuk V, Niyompattama A, Teosirimongkol C, Sriwanichrak K. False-positive serum and bronchoalveolar lavage Aspergillus galactomannan assays caused by different antibiotics. Scand J Infect Dis. 2010;42:461–8.PubMed
20.
Brodoefel H, Vogel M, Hebart H, et al. Long-term CT follow-up in 40 non-HIV immunocompromised patients with invasive pulmonary aspergillosis. Am J Roentgenol. 2006;187:404–13.
21.
Buchheidt D, Baust C, Skladny H, et al. Detection of aspergillus species in blood and bronchoalveolar lavage samples from immunocompromised patients by means of 2-step polymerase chain reaction: clinical results. Clin Infect Dis. 2001;33:428–35.PubMed
22.
Burghi G, Lemiale V, Seguin A, et al. Outcomes of mechanically ventilated hematology patients with invasive pulmonary aspergillosis. Intensive Care Med. 2011;37:1605–12.PubMed
23.
Busca A, Locatelli F, Barbui A, et al. Usefulness of sequential Aspergillus galactomannan antigen detection combined with early radiologic evaluation for diagnosis of invasive pulmonary aspergillosis in patients undergoing allogeneic stem cell transplantation. Transplant Proc. 2006;38:1610–3.PubMed
24.
Caillot D, Casasnovas O, Bernard A, et al. Improved management of invasive pulmonary aspergillosis in neutropenic patients using early thoracic computed tomographic scan and surgery. J Clin Oncol. 1997;15:139–47.PubMed
25.
Caillot D, Couaillier JF, Bernard A, et al. Increasing volume and changing characteristics of invasive pulmonary aspergillosis on sequential thoracic computed tomography scans in patients with neutropenia. J Clin Oncol. 2001;19:253–9.PubMed
26.
Caillot D, Latrabe V, Thiébaut A, et al. Computer tomography in pulmonary invasive aspergillosis in hematological patients with neutropenia: a useful tool for diagnosis and assessment of outcome in clinical trials. Eur J Radiol. 2010;74:e172–5.PubMed
27.
Caillot D, Mannone L, Cuisenier B, Couaillier JF. Role of early diagnosis and aggressive surgery in the management of invasive pulmonary aspergillosis in neutropenic patients. Clin Microbiol Infect. 2001;7 Suppl 2:54–61.PubMed
28.
Camus P, Costabel U. Drug-induced respiratory disease in patients with hematological diseases. Semin Respir Crit Care Med. 2005;26:458–81.PubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree