Fig. 4.1
Etiologies of dyspnea in cancer patients. BOOP bronchiolitis obliterans organizing pneumonia
Pneumothorax
Pneumothorax may be classified as spontaneous, iatrogenic, or tension pneumothorax or hydropneumothorax. Etiologies for pneumothorax include procedural complications (pleural or central venous access), pleural metastatic disease, infections (necrotizing pneumonia, Pneumocystis jirovecii), therapy sequelae (radiation, radiofrequency ablation), and underlying chronic lung disease. Authors have reported spontaneous pneumothorax associated with successful chemotherapy for lung metastasis.
Clinical manifestations of pneumothorax include acute dyspnea, hypoxemia, and subcutaneous emphysema. Pneumomediastinum and pneumoperitoneum can be attributed to spontaneous pneumothorax, as well.
Treatment of pneumothorax is based on radiologic criteria and clinical symptoms. The diagnosis may be confirmed using chest radiography, computed tomography (CT), or ultrasonography. Interventions include supplemental oxygen, aspiration of air, and placement of a chest tube or surgery. Small pneumothoraces (less than 10–15 % of hemithorax) in stable patients can be observed clinically and with serial chest radiographs. In comparison, symptomatic patients with larger pneumothoraces (greater than 15 % of hemithorax) should undergo interventions. If a pneumothorax does not improve, including radiologically, after intervention, the physician should evaluate the patient for other causes of dyspnea.
Pleural Effusion
Malignant pleural effusion can occur with all types of cancer and usually suggests advanced disease (Shannon et al. 2010b). Malignancies that commonly cause malignant pleural effusions include lung, breast, ovarian, gastrointestinal cancers and lymphoma. Cancer can metastasize hematogenously or contiguously. Other etiologies for malignant pleural effusion include tumor emboli to visceral pleura and tumor seeding from visceral to parietal pleura (Uzbeck et al. 2010). Pleural metastases do not necessarily result in pleural effusion in all cases, and not all pleural effusions in cancer patients are caused by malignancy. Confirmation of malignancy via pleural fluid cytology or pleural biopsy is recommended.
Clinical manifestations of malignant pleural effusion range from shortness of breath with exertion, chest pain, and cough to acute respiratory failure. Standard 2-view chest radiographs provide the most useful information, including the degree of hemithorax opacification, mediastinal shift, and other pulmonary processes (Fig. 4.2). Lateral decubitus films may help identify free-flowing pleural fluid collection. However, bedside ultrasound is used more frequently for this. A CT scan may provide additional information, including that on pleural thickening, loculation, a concomitant mass or endobronchial obstruction, thromboembolic disease, and pneumonia.
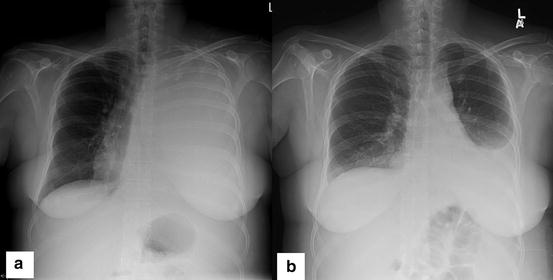

Fig. 4.2
(a) Chest radiograph of a 48-year-old woman with metastatic endometrial cancer demonstrating left hemithorax opacification. Of note is a shift of the trachea to the right owing to massive pleural effusion. (b) Chest radiograph showing subsequent improvement in aeration of the left hemithorax in the patient after placement of a pleural catheter
Emergent thoracentesis is often required when the patient has a contralateral shift in the mediastinum and/or respiratory distress. Thoracentesis may not relieve respiratory symptoms when the patient has a concomitant respiratory process such as an airway obstruction, a space-occupying mass, lymphangitic spread, a pulmonary embolism, or an infection.
Although the majority of malignant pleural effusions are exudative, 2–5 % of them may be transudative. Pleural fluid should be submitted for cell counts with differentials, chemistry analysis (glucose, protein, lactate dehydrogenase, cholesterol, triglyceride, and hematocrit measurement), and cytology. Also, flow cytometry data may be obtained for patients with underlying hematologic malignancies. Diagnostic thoracentesis is recommended for a new or persistent pleural effusion without a clear etiology (Fig. 4.3).
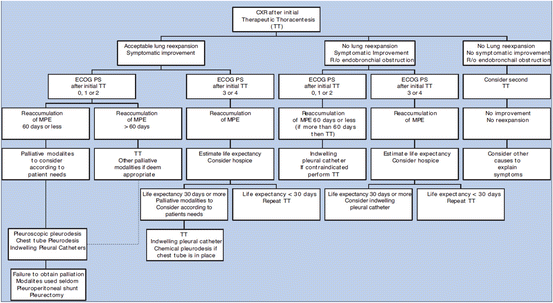

Fig. 4.3
Management of malignant pleural effusions. [Taken from Shannon VR et al. Respiratory complications. Chapter 131 in: Cancer Medicine, 8th edition. Hong WK, Bast RC Jr, Hait WN, Kufe DW, Pollock RE, Weichselbaum RR, Holland JF, Frei E III, eds. Shelton, CT: PMPH-USA, LTD. Used with permission from the People’s Publishing House-USA.]
Therapeutic options for pleural effusion include repeat thoracentesis, placement of an indwelling tunneled pleural catheter, use of a chest tube with talc pleurodesis, and medical thoracoscopy (Fig. 4.4) or video-assisted thoracic surgery with talc pleurodesis.
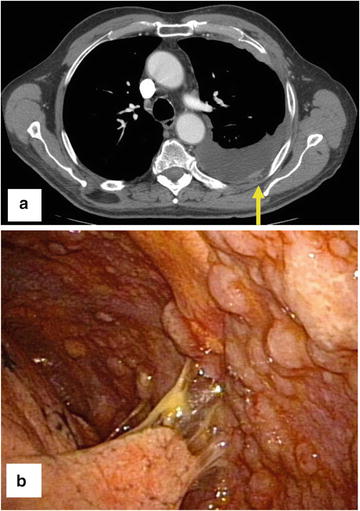

Fig. 4.4
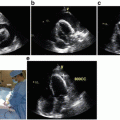
An 83-year-old patient presented with a recurrent exudative pleural effusion of unclear etiology. (a) CT scan revealing the pleural effusion with nodularity in the pleura (yellow arrow). Ultrasonography revealed a moderate collection of pleural effusions with an atelectatic lung. (b) Pleuroscopic image revealing metastatic tumor deposits throughout the pleura consistent with mesothelioma
Thoracentesis
Thoracentesis is the preferred method of diagnosis of a new pleural effusion. Ultrasonographic guidance is almost always used, and it has become the standard of care. Repeat thoracentesis is often recommended for patients with recurrent effusions, which are those recurring at least 60 days after the first pleural procedure; life expectancies shorter than 1 month; and poor performance status. Although the effects of thoracentesis may only be temporary, added discomfort or morbidity resulting from more aggressive procedures may make those procedures less desirable than thoracentesis given the clinical scenario. The American Thoracic Society suggests removal of up to 1.5 L of fluid at a time or until symptomatic.
Indwelling Tunneled Pleural Catheter
Indwelling tunneled pleural catheters may be placed in patients with recurrent (often malignant) pleural effusion (Fig. 4.5). These catheters may be placed in the outpatient or inpatient setting. The care of the catheter is usually performed by patients’ family members and/or home health services. Catheters are typically drained daily or 3 times a week until pleurodesis is achieved. The catheter is usually placed anteriorly; however, the placement may vary in patients with loculated fluid or chest wall deformities. Local anesthesia with two incisions (pleural and tunnel entry sites) is part of the catheter placement procedure. The complication rate for tunneled indwelling pleural catheters ranges from 2.0 % to 9.1 %, and complications include bleeding, cellulitis, empyema, catheter malfunction or blockage, chest pain, pneumothorax, and tract metastasis. A pleural catheter may be placed while the patient is receiving chemotherapy; with patient and family education, this is a reasonably safe option.
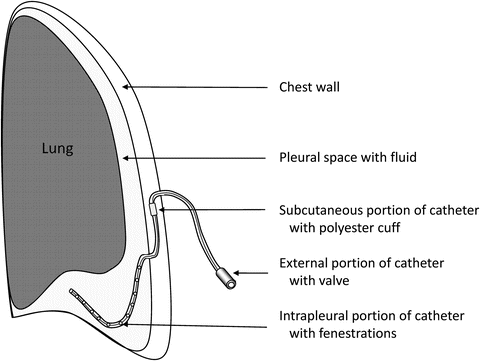

Fig. 4.5
Diagram of an indwelling pleural catheter [Courtesy of Dr. Rodolfo Morice, MD Anderson]
Pleurodesis with Chest Tube and Thoracoscopic Interventions
A tube thoracostomy is placed with or without sedation, and an inpatient stay is necessary for instillation of sclerosing agents . Medical thoracoscopy involves advancing a pleuroscope into the pleural space under sedation for diagnostic sampling of the pleura and/or instillation of sclerosing agents. Complications of tube thoracostomies include subcutaneous emphysema, fever, pain, sepsis, bleeding, and shock, but they are rarely reported. Video-assisted thoracic surgery is performed in the operating room, and it allows for the use of more extensive therapies. Chemical pleurodesis may result in fever and pain, which may prolong hospitalization. Also, authors have reported acute respiratory failure in patients with tube thoracostomies. Talc is the agent most commonly used with thoracostomies; doxycycline, bleomycin, and other experimental agents also have been used.
In summary, the diagnostic and therapeutic regimens for pleural effusion vary based on patient symptoms, the underlying malignancy, and concomitant pulmonary issues. The approach to definitive management of pleural effusion is based on the cancer stage, the prognosis, patient preference, available resources, and the site of care.
Radiation-Induced Lung Injury
Radiation therapy for cancer is effective because it destroys cancer cells, but it can be equally, if not more, damaging to adjacent normal tissue. Pneumonitis is a well-recognized potential complication of irradiation of the thoracic area (Graves et al. 2010). Damage to pneumocytes and endothelial cells may cause acute inflammation, and chronic injury reflects the resulting interstitial fibrosis.
The amount of ionizing radiation required to cause pneumonitis is about 20–25 Gy, whereas the intent-to-cure dose is considerably higher. An important consideration is the planned schedule for radiation administration. For example, daily treatments of 2.67 Gy carry significant risk, whereas fractions of 1.8–2.0 Gy are better tolerated. The total dose and volume of irradiated lung are obviously important, but other variables, including previous radiation therapy, individual genetic susceptibility, and underlying chronic lung disease, should be taken into consideration. Certain chemotherapeutic agents (e.g., bleomycin, vincristine, doxorubicin, cyclophosphamide, actinomycin D, gemcitabine, taxanes) may predispose patients to radiation pneumonitis, which may occur as early as 6 weeks and up to 1 year after radiation therapy. Both acute and chronic radiation pneumonitis may be insidious in development and may unfold in a continuous, sequential fashion.
The acute phase of radiation pneumonitis is characterized by nonproductive cough in its mildest form. With increasing severity, the cough becomes refractory, dyspnea becomes more pronounced, and oxygen therapy for hypoxia may become necessary. Fever may be present, as well. Radiologic findings include diffuse haze and indistinct vascular margins with sharp cut-off from surrounding unaffected tissue. A radiographically diffuse pattern outside the irradiated field of the lung develops, indicating a hypersensitivity-like reaction. A well-recognized rare presentation is radiation recall pneumonitis, which develops upon challenge with medications such as anthracyclines, taxanes, tamoxifen, and gemcitabine. The radiation therapy may have been complete weeks to months prior to challenge.
Corticosteroids may be used for radiation pneumonitis and tapered as determined by the clinical response. Treatment of very late-stage disease with steroids has demonstrated no significant clinical benefits. Researchers developed intensity-modulated radiation therapy and proton therapy to reduce the irradiation of normal tissue, but radiation pneumonitis can still occur in patients who undergo these two modalities . Pharmacologic therapy with amifostine has some cytoprotective properties.
Aspiration Pneumonia
Swallowing dysfunction in cancer patients can be caused by the underlying malignancy or its treatment. Any disturbance in the swallowing mechanism involving the central or muscular phase can result in aspiration of oropharyngeal contents into the lung.
Aspiration can lead to pneumonitis or pneumonia in the acute or chronic phase . Aspiration pneumonia may be overt or silent, and it often mimics community- or hospital-acquired pneumonia. Malignancies of the head and neck, esophagus, and lung often predispose patients to aspiration pneumonia. Tumor site, age, chemoradiation with mucositis, strictures, and surgery-related complications such as denervation, scar tissue formation, and reconstruction are factors affecting the development of aspiration pneumonia (Raber-Durlacher et al. 2012). Primary brain tumors and metastatic brain lesions can cause aspiration pneumonia via a centrally mediated mechanism.
If aspiration is severe enough to cause respiratory failure, it can be fatal. Pneumonia and pneumonitis lead to cough with or without fever. When examining patients’ medical histories, physicians should focus on their oncologic histories, presence of cough after swallowing meal boluses, sensation of dysphagia, perception of excessive viscous or paucity of saliva, examination findings revealing poor dentition making patients prone to infection, and complaints of nausea with or without vomiting. A modified barium swallow is the gold standard for evaluation of dysphagia and is performed with ingestion of measured quantities of liquids and solids. Fiber-optic endoscopic evaluation of swallowing is an alternative method of examination of residuals, laryngeal penetration, and aspiration.
Cultures often reveal oropharyngeal flora with aerobic and anaerobic bacteria in patients with aspiration pneumonia, and antimicrobial treatment aimed at these pathogens is usually effective. Definitive treatment of aspiration pneumonia involves prevention of future aspiration episodes using swallowing exercises, feeding gastrostomies, or esophageal stricture correction. Prevention of dysphagia may involve changes in dosing and delivery of radiation, close adherence to recommendations for early postradiation swallowing recovery (Rosenthal et al. 2006), use of cytoprotectants, and reconstructive surgery with tumor resection.
Hemoptysis
Hemoptysis may occur in cancer patients, mainly owing to the underlying malignancy or metastatic disease or as a complication of therapy (Shannon et al. 2010b). Massive hemoptysis carries a high mortality rate, and death generally results from asphyxiation rather than blood loss. In quantitative terms, massive hemoptysis is described as loss of at least 600 mL of blood in a 24-hour period.
Bleeding from a pulmonary malignancy can be caused by endobronchial disease or lesions distal to the airway. Bronchiectasis, invasive pulmonary infections (such as those with Aspergillus species), thromboembolic disease, and arteriovenous malformations are other possible etiologies of hemoptysis. Chemotherapy with agents that affect coagulation parameters and platelets (e.g., bevacizumab) may also predispose patients to bleeding. Diffuse alveolar hemorrhage may occur in patients after bone marrow transplantation and in leukemia patients with refractory thrombocytopenia. These patients may also present with hemoptysis. Treatment of hemoptysis in this subset of patients includes optimization of hematologic parameters and empiric steroids.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree