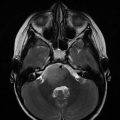
Fig. 20.1
Target contouring of a large skull base chordoma with extensive extracranial extension following subtotal, transnasal/transsphenoidal resection. Gross tumor volume (GTV) includes residual gross disease, equivocal areas as per imaging, and potential high-risk microscopic disease (red). Clinical target volume (CTV) includes GTV, potential microscopic areas but respecting anatomic barriers and considerations of infiltrative potential, as well as nasal surgical route. Note: brain parenchyma typically not included in the CTV except an outer “rim”
20.5 Tumor and Target Volume Prescription Doses
Most available data in clinical practice are based on 1.8–2.0 GyRBE dose fraction regimen, assuming a relative biologic effectiveness (RBE) factor of 1.1 of protons versus photons.
Although no formal dose escalation study has been conducted, there appears to be a radiation dose–response curve for chordomas. This conclusion is supported by the inferior results associated with photon therapy with doses of 66–68 Gy in contrast to the vast majority of proton data, where GTV prescription doses are in excess of 72 GyRBE. It appears that the minimum dose for very small GTV targets should be 72 GyRBE, for small to medium size tumors approximately 75–76 GyRBE and for large targets up to 78–80 GyRBE.
For the clinical target volume (CTV), doses analogous to general sarcoma principles for low-risk microscopic disease are used, i.e., doses between 50 and 54 GyRBE. Figures 20.2 and 20.3 depict typical isodose distributions that can be accomplished with spot scanning technology for locally advanced skull base chordomas.
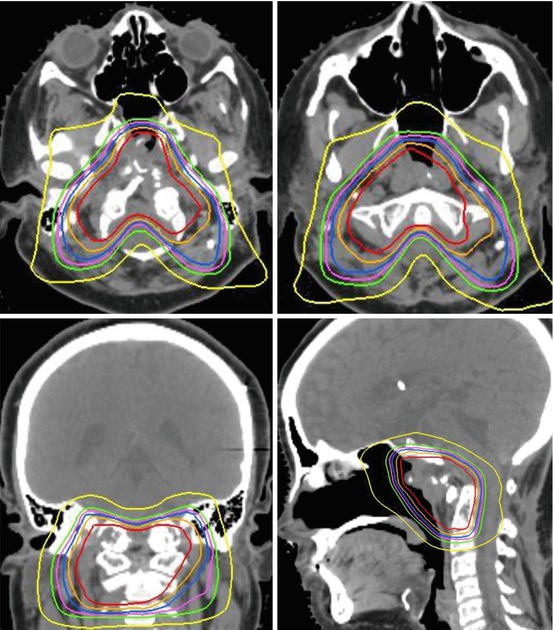
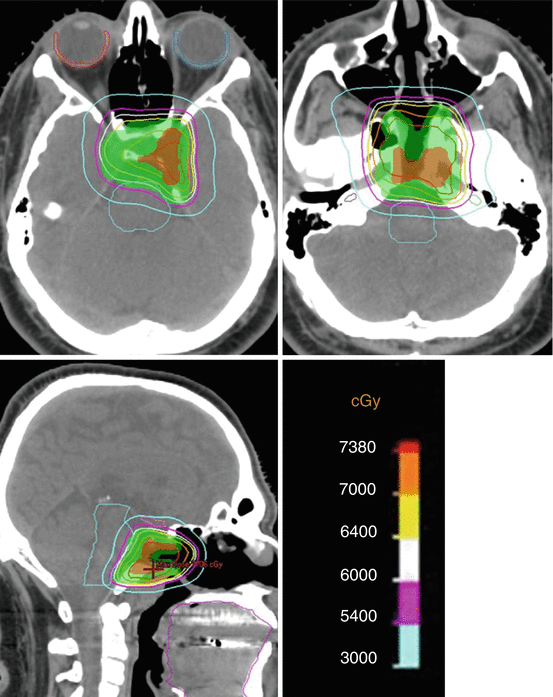

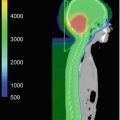
Fig. 20.2
A 13-year-old boy with extensive chordoma of the clivus with extracranial extension. Status post 2 subtotal resections. Proton therapy of GTV to 75.7 GyRBE and CTV to 54 GyRBE at 1.8 GyRBE dose per fraction

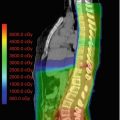
Fig. 20.3
Chordoma in a 19-year-old male. Status post-transnasal subtital resection. Contour display of PTV of CTV (green shaded volume) and GTV (red volume). Proton therapy prescription of CTV to 54 GyRBE (green shaded volume = PTV of CTV). GTV to 73.8 Gy (RBE) at 1.8 GyRBE dose per fraction
Although chondrosarcomas are very rare in children compared to chordomas, it is worthwhile to briefly outline some differences, as they have been established in young adults and adults in general: No prospective trials have been undertaken between chordomas and chondrosarcomas, it appears that higher local control rates can be obtained in chondrosarcomas with total prescription dosage ranging between 70 and 76 GyRBE. Most published data indicate actuarial local control rates >80% and up to 95% at 5-years and even 10-year local control rates >80% for chondrosarcomas, which is even more remarkable considering the higher percentage of chondrosarcoma patients compared to chordoma patients with medium- to large-size disease since they are relatively unresectable compared to chordoma due the inherent calcified nature. There is presently no analysis available that indicates a dose differential between smaller size and larger size chondrosarcomas since local control rates appear equally high. In clinical practice, the GTV dose ranges between 70 GyRBE for small residual disease and 75 GyRBE for large-volume disease. If the patient is referred postoperatively for no residual disease, then presumably high-risk areas could be treated to approximately 66–68 GyRBE only.
20.6 Organs at Risk, Special Dose Considerations
A matter of significant debate remains the critical OAR constraints since the maximum permissible dosages used in some proton centers exceed the typical dose constraints used in conventional photon therapy. The general low risk of high-grade adverse events reported after proton therapy with high critical OAR constraints is likely supported by a dose–volume effect, i.e., the sharp dose fall-off within an OAR. This is mentioned in the QUANTEC report for OAR recommendations where it is explicitly stated that “for dosages at the level of 60 Gy no data are presently available on the dose volume effect” (Marks et al. 2010).
The ALARA (As Low As Reasonably Achievable) rule of dose exposure applies to each patient. If a tumor is not in immediate vicinity, abutting or compressing an OAR, then every effort should be made to keep this OAR at lower dose levels compared to situations with tumor abutment. The following OAR constraints are presently used by the author and reflect general guidelines only that need to be carefully adjusted to the individual patient and situation. The author allows a maximum dose to 0.01 cm3 up to 60 GyRBE to either optic chiasm or one of the optic nerves. Dose levels up to 60 GyRBE to single optic structures have resulted in the majority of publications in severe adverse event rates, i.e., grade 3 or higher toxicity, of optic structures of less than 1% (Munzenrider and Liebsch 1999). One should consider that inherently the dose tolerance of optic structure is likely lower if the patient has preexisting visual impairment. In addition, OAR constraints to optic structures should be reduced if the patient has monocular vision. In the author’s opinion, a significant risk of complete blindness is unacceptable and renders the patient unsuitable for proton therapy. Ethical considerations apply to the use of high-dose radiation analogous to surgical resection. If complete tumor resection would require resection of the optic chiasm, such tumor is deemed “unresectable” by the neurosurgeons. Radiation dose to the optic structures that are associated with a high risk of complete blindness should not be considered.
With regard to the brainstem, the author uses a surface dose constraint of 64 GyRBE and a brainstem center dose of 53 GyRBE, as they have been developed early on by the group at the Harvard Cyclotron Laboratory and Massachusetts General Hospital (MGH/HCL). The surface dose can be defined as meaningful volume (e.g., 0.5 cm3) and as the isodose line that is repeatedly permitted to touch the brainstem surface. The low brainstem center dose ascertains a sharp dose fall-off inside the brainstem. Based on the publication in 1999 by Debus who reported the MGH/HCL experience (Debus et al. 1999), this approach has resulted in exceedingly low rate of brainstem toxicity in adults and children with skull base chordomas and chondrosarcomas. At most there are only anecdotal reports of severe brainstem injuries in other large series.
Grade 3 or higher brain parenchymal toxicities remain the highest single organ severe adverse event rate reported in high-dose skull base tumor treatments. Several publications have reported rates of approximately 4–6% for adult patients. Despite significant efforts of predictive analysis, at present no generally agreed upon OAR constraints have proven to reduce this rate significantly. A constraint of 72 GyRBE to <2 cm3 of volume per individual lobe appears practical. The medial temporal lobes are at the highest risk when treating superior skull base tumors, which may be due to the competing adjacent OARs. In order to avoid the optic chiasm, optic nerves and brainstem require use of lateral fields that either range out in the contralateral medial temporal lobe with passive scattering technology or place repeated highly weighted spots in the medial temporal lobes with active spot scanning. These issues illustrate the need of additional fields besides lateral beams for upper skull base lesions to minimize the risk of temporal lobe necrosis especially when the PTV extends into this area.
20.7 Normal Tissue Constraints Versus Target Coverage
GTV prescription doses for either residual chordoma or chondrosarcoma exceed the majority of normal tissue tolerances; hence, the specification of OAR constraints significantly impacts on the general ability to cover the GTV volume and defines the steepness of the shoulder region of the target dose–volume histograms. Figure 20.4 illustrates a patient treated to a total target dose of 73.6 Gy (RBE) for relatively small residual disease chordoma of the upper clivus with no brainstem compression and no optic apparatus abutment—a rather favorable situation. Treatment plans were generated using identical contours and prescription dose with either the author’s OAR constraints of 60 and 64 GyRBE or the more common limits of 55 and 55 GyRBE to the optic chiasm and brainstem surface, respectively. The resulting GTV DVHs are displayed in Fig. 20.5. The use of lower OAR constraints alone resulted in a significant reduction of therapeutic dose for this small and favorable GTV. Although the exact coverage reduction is dependent on institution specific equipment, it exemplifies qualitatively the importance for each treating physician to carefully consider their OAR constraints and integrate published proton center constraints into their plans. Using low OAR constraints might allow a lower risk of high-grade complications but might also considerably reduce the patient’s chances of local control and potentially eradicate any gain in tumor control probability by protons over conventional photon therapy.
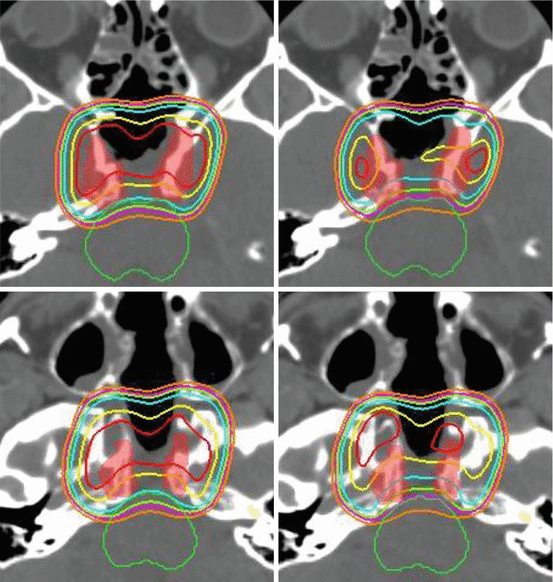
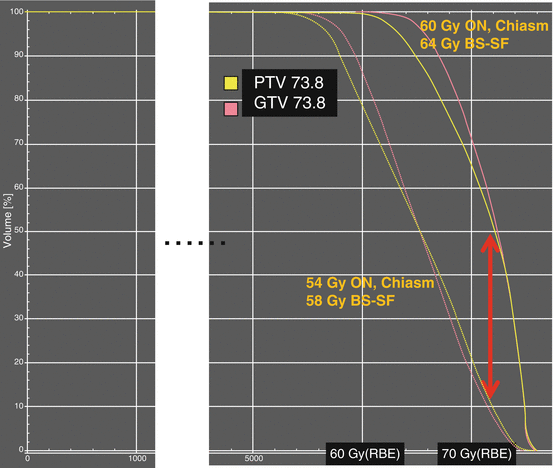

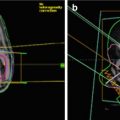
Fig. 20.4
Influence of decreasing OAR constraints on resulting target coverage. Prescription to GTV 74 Gy(RBE). OAR constraint for optic nerves and chiasm of 60 Gy(RBE) (Panel a) versus 54 Gy(RBE) (Panel b). OAR constraint for brainstem surface of 64 Gy(RBE) (Panel c) versus 58 Gy(RBE) (Panel d). Isodose display ranging from 54 (orange) to 64 (blue) to 74 Gy(RBE) (dark red)

Fig. 20.5
Influence of OAR dose constraints on target coverage according to dose–volume histogram
20.8 Prognostic Factors
In general for skull base tumors, chordomas need to be distinguished from chondrosarcoma since they are two distinctly different histologies with different prognosis. Low-grade chondrosarcomas by and large have a better prognosis following high-dose proton therapy than chordomas. Since the majority of skull base chondrosarcomas can be controlled long term with proton therapy, little information is available on prognostic factors. There is no reliable information available if large size, unresectable skull base chondrosarcomas have a lesser prognosis compared to small residual disease following high-dose proton therapy.
In contrast, for low-grade chordomas various prognostic factors have been identified in the adult population that determines the likelihood of accomplishing local control and long-term survival. Although those have not been specifically validated for the pediatric population, this still serve as helpful guidelines.
- (a)
Size of residual disease plays an important role: Small-volume disease (defined as approximately 25–30 cm3) appears to be correlated with an excellent long-term prognosis of >90% at 5 years. The chances drop significantly from small-volume to mid-size (approximately 50 cm3) to larger volumes.
- (b)
Compression or tumor abutment of dose-limiting OARs at the time of proton therapy is correlated with reduced chances of obtaining permanent local control—likely due to the dose gradient between maximum permissible OAR dose and tumoricidal prescription dose.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree