Fig. 1
A typical passive scatter treatment nozzle with associated hardware
In contrast, intensity—modulated proton therapy obviates the need for physical beam—shaping devices. In IMPT a small (3–5 mm) beam of protons is electromagnetically scanned over the target volume, with dose being deposited in effect “layer by layer”, with the typical layer thickness being on the order of 1 mm. Bragg Peak placement is achieved by dynamically varying the energy of the proton beam (Lomax 1999). Thus, treatment delivery is analogous to the operation of a 3-Dimensional printer that creates a complex, solid object by precisely depositing varying thicknesses of material. With IMPT, treatment dose can be optimized to the target itself and what is more, the delivery of differential radiation doses within the target becomes both feasible and easily achievable. In addition, since the beam manipulation is performed electromagnetically and not by patient-specific physical devices, IMPT plans can be rapidly altered (often within 24 h) to reflect changes in patient anatomy and tumor configuration. The first IMPT treatment systems were developed in the early 2000s (Lomax et al. 2004), and this proton treatment method became available in the United States in 2008. Rapid advances in this technology have led to the construction of “IMPT-only” treatment facilities and indeed the vast majority of recently commissioned proton centers, and those under construction, are designed to employ this technology as their sole means of proton beam treatment delivery. A diagram of a typical IMPT treatment nozzle is shown in Fig. 2.
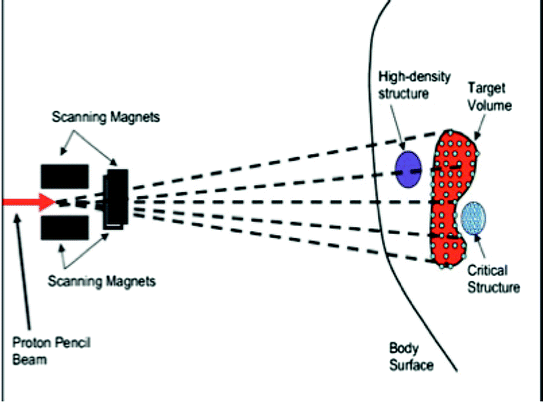

Fig. 2
A diagram of a typical IMPT treatment nozzle
3 Treatment Planning
Whether employing PSPT or IMPT, all proton beam therapy planning, like modern X-ray therapy planning, is based upon creating a three-dimensional reconstruction of the target and adjacent normal tissues. In general, the patient positioning and immobilization techniques which are utilized in X-ray therapy are equally applicable to proton beam treatment. Similarly, the concepts of gross tumor volume (GTV) and clinical target volume (CTV) are also identical to those used in IMRT, however, the unique physical characteristics of a proton beam result in a modification of the X-ray therapy planning target volume (PTV) into either a beam—specific PTV (in the case of PSPT), or a “Scanning Target Volume” in IMPT (Lomax et al. 2004). One of the primary differences between proton therapy and X-ray therapy dosimetry lies in the uncertainty as to exactly where a proton of any given energy will come to a stop. This “range uncertainty” is partly due to the need to convert tissue densities obtained from CT (which are quantified as Hounsfield Units) to proton stopping power; this process typically adds a range uncertainty of up to 3% to the precise location at which any given proton will come to rest (Lomax 2009). Since the protons range is also significantly affected by tissue density, it is a common planning practice to avoid to the greatest extent possible beam arrangements which traverse anatomic structures (such as small or large intestine) which vary widely in density and in anatomic location. This partly explains the reason that the vast majority of prostate cancer patients treated with protons have their treatment delivered through a left and right lateral field as this field arrangement minimizes density uncertainties within the beam path.
Another dosimetric issue which is unique to proton and other heavy charged particle beam treatment is the need to account for the Relative Biologic Effectiveness (RBE) of the proton so as to proscribe a radiation dose whose biologic equivalent is accurately linked to known doses and risks of normal tissue injury established by X-ray therapy. In general, a relative biologic effectiveness (RBE) of 1.1 is assumed for protons as compared to megavoltage X-rays and although this approximation is undoubtedly an oversimplification it has clinically proven to be an accurate value for predicting both disease response and the risk of normal tissue injury.
The clinical implementation of IMPT has, in a fashion analogous to what was seen with the implementation of IMRT, resulted in the introduction of additional complexity into the treatment planning process. For one thing, the ability to deliver differential radiation doses within any given target volume means that (again, in a fashion identical to IMRT) in effect a proton “fluence map” is created. Not only does this result in quality assurance needs which are identical to those utilized in IMRT, combining this fluence map with the confounding factors of proton range uncertainty as well as patient positional uncertainty has led to the introduction of a property known as “robustness” in IMPT planning (Lomax 1999, 2008; Lomax et al. 2004). Robustness is in effect a probability analysis which graphically displays (typically by means of dose—volume histograms) the likely range of dose distributions for any given beam arrangement and the probability that any one given treatment plan will accurately and reproducibly irradiate the target structure while simultaneously minimizing radiation dose to normal tissues. Robustness is influenced by a number of factors including the degree of patient immobilization, the depth of the target, the density of the tissues proximal to the target, and whether or not the patient is being treated with a single—field optimization (in which all proton beams “see” the entirety of the target) or a multi-field optimization (in which any one given beam may only “see” a portion of the target, with the summation of all beams resulting in the desired radiation dose to the target). Because of its favorable anatomic location IMPT prostate plans tend to be very robust although they are still sensitive to factors such as patient rotation (which may alter the density of bone between the skin surface and the prostate) and the presence of distensible organs such as the bladder or rectum within the beam path.
4 Early Proton Beam Treatment Results
The ability to use proton beam therapy to treat deep organs was and remains greatly dependent on the concurrent development of cross-sectional imaging technology (CT, MRI) and modern computers, hence it is not surprising that proton beam therapy of prostate cancer did not commence until the late 1970s. Beginning in 1977, Shipley and associates at the Massachusetts General Hospital (MGH) initiated a Phase I trial in which proton beam radiotherapy was used to deliver a boost dose to patients with locally advanced disease who were also receiving photon radiotherapy. At that time, this boost dose was felt to be over and above what could be safely given with existing 2-Dimensional photon technology. Seventeen patients with stage T2–T4 disease received a perineally-directed proton beam boost of 2000–2600 rads (given at a rate of 180–200 rads per day) which was proceeded by treatment of the prostate and pelvis to a dose of 5040 rads with 10 MV photons delivered as a four-field box. A perineal approach was mandated because this was the only anatomical pathway that allowed the 160 meV proton beam generated by the Harvard Cyclotron to reliably encompass the entire prostate gland. Acutely, the treatment was well tolerated and after a follow up period ranging from 12 to 27 months no severe late rectal reactions were noted (Shipley et al. 1979).
These favorable toxicity results led directly to the initiation of a prospective randomized trial designed to test the benefits of proton beam dose escalation in patients with locally advanced disease. Patients with stage T3–T4 tumors were chosen as it was felt that this group stood to benefit the most from dose escalation. All patients received 50.4 Gy to the prostate and pelvis with megavoltage photons. They were then randomized to receive either an additional 16.8 Gy of photons (for a total prostate dose of 67.2 Gy) or 25.2 GyE of protons for a total prostate dose of 75.6 Gy. Adjuvant hormonal therapy was not permitted. The limited availability of the Harvard Cyclotron significantly impacted patient accrual; nonetheless, two hundred and two patients were eventually enrolled, with one hundred and three being treated in the high dose proton boost arm and ninety-nine in the standard dose arm.
With a median follow up of 61 months there were no differences seen in overall survival, disease-specific survival, total relapse-free survival, or local control between the arms. Patients with high-grade tumors who were treated on the high dose arm did experience a trend improvement in local control at five and eight years (92 and 77% vs. 80 and 60%, p = 0.89). Patients whose digital rectal exams normalized following treatment and who underwent subsequent prostate biopsy revealed a lower positive biopsy rate in the high dose arm (28 vs. 45%) and, perhaps most surprisingly, the local control rates for patients with Gleason grade 4–5 tumors (57 patients total) were significantly better at five and eight years in the high dose patients (94 and 84% vs. 68 and 19%, p = 0.0014). High dose treatment was associated with an increase in late grade 1–2 rectal bleeding (32 vs. 12%, p = 0.02) (Shipley et al. 1995).
Some critics have repeatedly and in my opinion incorrectly cited these results as evidence that proton-beam dose escalation is of doubtful utility. It should be noted that the patients treated in this trial were at a high risk of not only local failure but also of distant failure and therefore one should not be surprised that overall survival was unaffected. In addition, patients with these adverse characteristics would not, if undergoing treatment today, receive radiotherapy as monotherapy and instead would be treated with a multi-modality approach. I believe that the two most important points learned from this study are (1) high dose radiotherapy did decrease local failure, and this decrease was most profound in those patients with the most aggressive tumors and (2) Dose-escalation by means of a perineal proton beam (an approach which has virtually universally been abandoned today as higher energy machines become available) could be performed safely with acceptable toxicity.
The improvement in local control seen with dose escalation prompted a very logical question: If patients with earlier stage disease who are less likely to have already experienced metastatic failure are treated with dose escalation will we see a positive effect on survival? This intriguing hypothesis has been tested in a prospective randomized multi-institution trial and its conclusions will be covered presently.
The completion in 1990 of the world’s first hospital-based proton treatment center at Loma Linda University Medical Center (LLUMC) marked the beginning of a transition in proton beam therapy from the research laboratory setting to that of clinical radiation oncology (Slater et al. 1988, 1992). Beginning in late 1991 prostate patients at LLUMC was treated on a clinical trial that set out to confirm the efficacy and toxicity data generated at MGH. Between December 1991 and December 1995 643 patients were treated to total prostate radiation doses of 74–75 GyE. Patients who were deemed to be at a low risk for occult nodal metastasis were treated with lateral proton beams alone while those who were felt to benefit from elective nodal radiation received 45 Gy to the pelvis with 18–23 MV photons delivered via a multi-field 3-D conformal technique. Patient characteristics are shown in Table 1 (Slater et al. 1998).
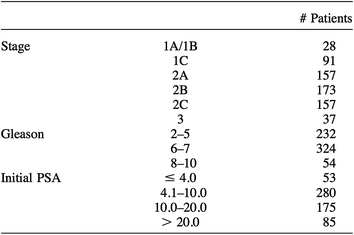
Table 1
LLUMC Patient Characteristics

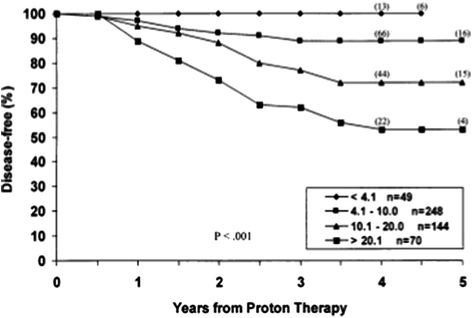
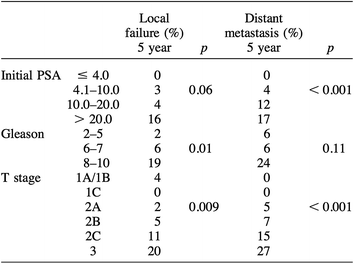
With a median follow up of 43 months, the overall biochemical disease-free survival (bNED) rate was 79% as per the original American Society for Therapeutic Radiology and Oncology (ASTRO) definition of three successively rising PSA values above a nadir equating to biochemical failure. The risk of biochemical failure was strongly dependent on the pre-treatment PSA with five-year bNED survival rates varying from 53% in patients with pre-treatment PSA’s of 20–50 to 100% with PSA’s of <4.1 (Fig. 3).
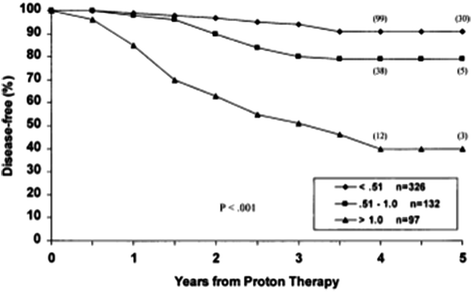
bNED survival was also significantly influenced by post-treatment PSA nadir (Fig. 4).
A multi-variant analysis of failure predictors demonstrated that initial stage, PSA, and Gleason Score were all strong predictors of biochemical failure at five years (Table 2).
 Similar to what was reported in the MGH trial, treatment was by and large well tolerated. Acute toxicity was minimal and all patients completed the prescribed course of radiotherapy. Proctitis remained the most common late toxicity with Grade 2 toxicity occurring in 21% of patients at three years; for the majority of patients this represented a single episode of rectal bleeding. No ≥Grade 3 GI toxicity was seen. Grade 2 GU toxicity (primarily gross hematuria) was seen in 5.4% of patients at three years, with two patients developing Grade 3 bladder toxicity. Interestingly, no significant difference in late toxicity was seen between those patients treated with protons alone and those receiving pelvic X-ray therapy. The excellent biochemical control rates and acceptable toxicity seen in this trial confirmed the earlier MGH data and led to the implementation of a prospective randomized dose escalation study in organ confined prostate cancer.
Similar to what was reported in the MGH trial, treatment was by and large well tolerated. Acute toxicity was minimal and all patients completed the prescribed course of radiotherapy. Proctitis remained the most common late toxicity with Grade 2 toxicity occurring in 21% of patients at three years; for the majority of patients this represented a single episode of rectal bleeding. No ≥Grade 3 GI toxicity was seen. Grade 2 GU toxicity (primarily gross hematuria) was seen in 5.4% of patients at three years, with two patients developing Grade 3 bladder toxicity. Interestingly, no significant difference in late toxicity was seen between those patients treated with protons alone and those receiving pelvic X-ray therapy. The excellent biochemical control rates and acceptable toxicity seen in this trial confirmed the earlier MGH data and led to the implementation of a prospective randomized dose escalation study in organ confined prostate cancer.

Fig. 3
bNED survival in relation to pre-treatment PSA

Fig. 4
bNED survival in relation to post-treatment PSA nadir
Table 2
Predictors of local/distant failure, initial LLUMC experience

A further update of the initial LLUMC experience was published in 2004. This study encompassed 1255 patients with stage T1–T3 disease who were treated with proton beam radiotherapy alone (i.e., no prior or concurrent hormonal therapy) to a dose of 74–75 GyE. As was seen in the earlier trial initial PSA, Gleason Grade, and PSA nadir were all strong predictors of bNED survival (Fig. 5a–c).
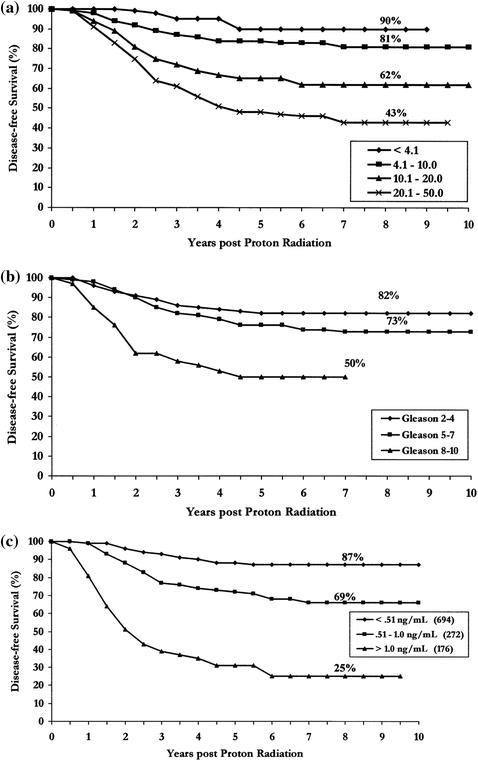
Treatment continued to be well tolerated with rates of RTOG Grade ≥3 GI/GU late morbidity of <1% (Slater et al. 2004).

Fig. 5
Effect of pre-treatment PSA on bNED survival (a) Gleason score on bNED survival (b) PSA nadir on bNED survival (c)
5 PROG 95-09 Trial
Beginning in 1996, LLUMC and MGH embarked on the Proton Radiation Oncology Group/American College of Radiology (PROG/ACR) 95-09 trial, a prospective, randomized dose-escalation study for patients with organ-confined prostate cancer. This study was designed to test the hypothesis that dose escalation from 70.2 to 79.2 GyE would result in a statistically significant decrease in local failure, biochemical failure, and overall survival. Eligibility criteria included stage T1b–T2b disease (as per the 1992 American Joint Committee on Cancer staging system), a PSA of ≤15 ng/ml, and no evidence of metastatic disease on imaging studies (bone scan, abdominal-pelvic CT scan). Gleason score was not an exclusion criterion, and no prior or concurrent androgen-depravation therapy was permitted. Pre-treatment patient characteristics are shown in Table 3.
Table 3




Pre-treatment patient characteristics PROG 95-09 trial
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree