13 Proteolytic Enzymes
13 Proteolytic Enzymes
 Introduction
Introduction
The oral administration of proteolytic enzymes (systemic enzyme therapy) has its origins in experience-based medicine and can be traced back, depending on the culture, to 1000-year-old records.
The enzyme-deficiency theory of cancer was articulated by the Scottish embryologist John Beard around the turn of the last century. His collected articles on the topic were published in book form in 1911 (1). This work was gradually forgotten. About 40 years ago, Max Wolf developed the concept of systemic enzyme therapy for oncology. The observation that tumor patients’ sera had reduced cytotoxic activity against tumor cells, which was restored after the addition of proteolytic enzymes, formed the basis of the therapy. In addition, it was noted that malignant tumors were more common in old age with a concurrent reduction in the production of pancreatic enzymes as well as hydrolytic activity in the serum.
This led to the attempts to restore the oncolytic activity of the serum through the administration of oral proteolytic enzyme mixtures. Proteolytic enzymes are large molecules that can be absorbed. They then disperse into different compartments in the body in various concentrations (Table 13.1).
Initial animal experiments showed that the growth of experimental tumors was reduced and that the hydrolytic activity of the serum was normalized (8).
During this time, the role of proteolytic enzymes in clotting and fibrinolysis was also discovered. Metastases and their organotropic tendencies were explained at the time by “fibrinolytic stickiness,” through which tumor cells docked onto other organs and evaded immune surveillance. Research in immunology in the following years increasingly brought to light explanations for the mechanisms of action of systemic enzyme therapy.
Pharmacological Examinations
• It was observed that tumor cells emitted factors that blocked the immune system (blocking factors). In this manner, tumor cells protect themselves by shedding surface structures (soluble antigens) from being recognized by antibodies and from the attack by cells of the immune system. Through soluble antigens, antibodies are caught, creating immune complexes that inhibit unspecified immune cells such as monocytes/macrophages and natural killer (NK) cells. The prevailing hypothesis at the time was that these blocking factors could be reduced through systemic enzyme therapy (9).
• Infiltrates of leucocytes during the rejection process provided indications of the interaction between macrophages and NK cells in the defense against foreign tissue and tumor cells. Aggregations of leucocytes are formed by means of adhesion molecules on tissues and immune cells.
For example, the adhesion molecule ICAM-1 on endothelial cells indicates an area of inflammation where leucocytes dock via the adhesion molecule LFA-1. Tumor cells can also carry adhesion molecules. The adhesion molecule CD44 plays a prominent role in metastasizing cells, because through them tumor cells are able to adhere to lymph nodes or organ tissues.
Various adhesion molecules belong to the so-called “superfamily” of immunoglobulins, such as antibodies, for example. Since the immune complex level was reduced after enzyme therapy, it was examined to what extent enzymes were able to alter comparable adhesion molecules. It was thereby demonstrated that not only was CD44 reduced, but the amount of adhesion molecules of the immune globulin super family on the surface was also reduced on target cells.
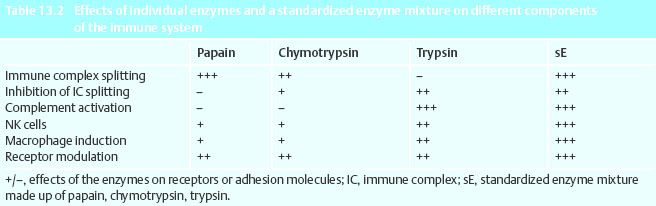
A contribution to the reduction of metastatic risk could therefore be seen in a reduction in the amount of CD44 on tumor cells. The increase in immune response against tumor cells plays a part in this (Table 13.2).
The blocking of cytotoxic activity of macrophages and NK cells through immune complexes has been examined in vitro. Through treatment of macrophages and NK cells with enzymes this blockage was reversed and cytotoxicity increased. This effect depends on the basic initial activity of the cells. Activated cells can only be stimulated to a small degree, or not at all. In tumor patients the immune system often shows a reduced activity because of tumor destructive therapy, but also because some tumor cells emit immune suppressive mediator substances (cytokines) such as transforming growth factor-beta (TGF-β) interleukin-4 (IL-4).
Changes of the in-vitro cytokine profile of NK cells and macrophages following administration of proteolytic enzymes were examined in isolated lymphocytes of plasmacytoma patients. Tumor necrosis factor-alpha (TNF-α) plays a critical role in the defense against tumor cells and is often inactive in tumor patients. In blood samples treated with proteolytic enzymes, the inactive TNF-α became tumoricidal again. When the isolated leucocytes were simultaneously incubated with proteolytic enzymes and proinflammatory cytokine interferon-γ, IL-6 was secreted.
Interleukin-6 (IL-6) belongs to the inflammatory cytokines, which indicates that enzymes regulate the interplay of inflammatory cytokines. In the last few years the Th1/Th2 scheme of immune response has moved to the center of research. The Th1 and Th2 immune reactions (T helper lymphocytes) are determined by different cytokine patterns, each representing another path of the immune response (3).
In studies looking at the pathogenesis of multiple sclerosis it was shown that proteolytic enzymes regulate the balance of Th1/Th2 cytokines. The cytokine TGF-β proved to be a key molecule. It regulates the repair of tissues, is an anti-inflammatory cytokine, and inhibits autoimmune diseases. In combination with an elevated level of TGF-β, one finds fibroses, scleroses (arterioscleroses), tumor growth, and metastases. Apparently the tumor growth and metastases through the immune suppressive effect of TGF-β is promoted. In a study on TGF-β -induced nephritic fibrosis in diabetes mellitus it was shown, that orally administered proteolytic enzymes inhibit fibrosis and simultaneously reduce TGF-β.
The reason for this is offered by a fundamental realization of systemic enzyme therapy: When proteolytic enzymes gain entrance into the blood circulation, they bind enzyme inhibitors, especially to α2-macroglobulin. During the binding of hydrolases/proteases to the α2-macroglobulin the molecule changes its form and characteristics. This α2-macroglobulin-hydrolase/protease complex can bind a variety of cytokines. If the complex is removed from the circulation, elevated cytokine levels can be reduced in this way (4).
 Experimental Studies
Experimental Studies
• In experience-based medicine proteolytic enzymes are orally administered for a reduction of side effects
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree