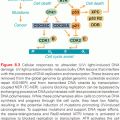
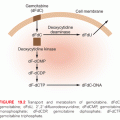
Preclinical Activity of Proteasome Inhibitors
Each of the three classes of inhibitors has a distinct chemical mechanism of proteasome inhibition.22 Peptide boronates form stable but reversible tetrahedral intermediates with the γ-hydroxyl (γ-OH) group of the catalytic N-terminal threonine of the proteasome active sites.23,24 β-lactones also interact with this γ-OH, but form a completely irreversible interaction.25 Similarly, peptide epoxy ketones form irreversible covalent adducts with the active site threonine but do so via a dual covalent adduction of γ-OH group and the free amine.26 This interaction is highly specific for N-terminal threonine-containing hydrolases and renders peptide epoxy ketones the most selective proteasome inhibitors yet described.27,28
The primary targets of these PIs within the constitutive and immunoproteasomes are the CT-L subunits, β5 and LMP7, respectively. Despite accounting for less than 50% of total protein turnover by the proteasome, these subunits are essential for cell survival.29 In MM cell lines, inhibiting both subunits (β5 and LMP7) is necessary and sufficient for tumor cell death.30 Cytotoxicity of other tumor cell types requires the inhibition of multiple active sites beyond the CT-L activity. The combination of inhibitors specific for either the T-L or C-L activities, which have no cytotoxic activity on their own, augments the cytotoxic potential of the CT-L–specific inhibitors.31,32
Given its status as the first proteasome inhibitor approved for marketed use, the antitumor potential and preclinical activity of other proteasome inhibitors have generally been compared to bortezomib.19 Carfilzomib showed equivalent antitumor activity to bortezomib in vitro against a panel of tumor cell lines under standard culture conditions but was >10-fold more potent at inducing tumor cell death when cells were exposed to drug for a 1-hour pulse, which mimics the pharmacokinetics of both compounds.33 MLN2238 (the active agent of ixazomib) was active in the same mouse models of human tumors as bortezomib, but demonstrated greater levels of proteasome inhibition in the tumors.34 In biochemical assays of proteasome activity, delanzomib had an identical potency and subunit activity profile to bortezomib, but in tumor cytotoxicity assays, potency relative to bortezomib was 2- to 10-fold less.35 In addition, delanzomib appeared to be less cytotoxic than bortezomib to normal cells and had a differential effect on cytokine release in bone marrow stromal cells, suggesting a different pharmacologic activity. Oprozomib is 10-fold less potent than carfilzomib in proteasome activity assays, but showed similar antitumor activity in mouse tumor models.36,37 Marizomib displayed greater potency against the non–CT-L active sites of the proteasome than bortezomib.38 Interestingly, this agent synergized with bortezomib in killing tumor cells in vitro.39 All of the second-generation inhibitors have shown activity in tumor cells made resistant to bortezomib and/or MM cells isolated from patients relapsed from bortezomib-based therapies.35,36,40–42
The inhibition of tumor cells with proteasome inhibitors induces cell death via the induction of apoptosis through death effector caspase activation.10 Although the mechanism underlying the induction of cell death remains to be fully elucidated, extensive research suggests a complex interplay of multiple pathways. PIs have been shown to affect the half-life of the BH3-only members of the Bcl-2 family, specifically BH3–interacting-domain death agonist (Bid) and Bcl-2 interacting killer (Bik).43 Moreover the BH3-only protein NOXA is upregulated at the transcription level by PIs.44–48 Proteasome inhibition also upregulates the expression of several key cell-cycle checkpoint proteins that include p53 (an inducer of G0/G1 cell-cycle arrest through accumulation of the cyclin-dependent kinase [CDK] inhibitor p27); the CDK inhibitor p21; mammalian cyclins A, B, D, and E; and transcription factors E2F and Rb.49,50 The transcription factor nuclear factor kappa B (NF-κB), an important regulator of cell survival and cytokine/growth factor production,51 is also affected by proteasome inhibition in multiple ways. The net effect on NF-κB signaling is not consistent across various assays and cell lines, and its relative importance in the antitumor effects of PIs remains unclear. Although it is interesting to note that patients whose myeloma harbor NF-κB–activating mutations (~20%) respond better to bortezomib than those without NF-κB–activating mutations.52–54 In MM cell lines, there is growing evidence that the major determinant of sensitivity to proteasome inhibition is the relative load of protein flux to the proteasome.55–57 These data suggest that induction of the terminal unfolded protein response may drive cell death. Whether proteotoxic stress induced cell death reflects sensitivity to proteasome inhibitors in other tumor types remains to be determined.
Pharmacokinetics and Pharmacodynamics of Proteasome Inhibitors in Animals
Following intravenous (IV) administration to animals and humans, proteasome activity is inhibited in a dose-dependent fashion within minutes; however, PIs such as bortezomib and carfilzomib are also rapidly cleared from circulation.55,56,58–61 Recovery of proteasome activity in animals occurs in tissues with a half-life of approximately 24 hours, mirroring the recovery time of cells exposed to sublethal concentrations of PIs in vitro and likely reflecting new protein synthesis.33,62
PROTEASOME INHIBITORS IN CANCER
Clinical Activity of Bortezomib
Bortezomib is typically administered on days 1, 4, 8, and 11 of a 3-week cycle either as an IV bolus or subcutaneous administration. Increasing doses of bortezomib inhibit proteasome activity in blood in a dose-dependent fashion, reaching a maximum of 74% inhibition at a dose of 1.38 mg/m2. Daily dosing schedules in animal studies have been associated with severe toxicity and have not been attempted in humans. In clinical trials, thrombocytopenia and peripheral neuropathy (PN) were common adverse events.20,63,64 Bortezomib has shown remarkable single-agent antitumor activity in a wide range of B-cell neoplasms, including MM, non Hodgkin lymphoma (NHL), and Waldenström macroglobulinemia (WM). In 2003, bortezomib was approved by the FDA for use as a single agent for the treatment of patients with MM following two prior therapies and who demonstrated disease progression with their most recent therapy. The primary efficacy data for this approval was derived from the SUMMIT trial in which 202 patients with heavily pretreated disease were treated with bortezomib at 1.3 mg/m2.65 In this trial, the overall response rate (ORR), defined as patients achieving at least a 50% reduction in serum or urine levels of the myeloma M protein, was 35%. This clinical trial was supported by the CREST trial, in which the activity of 1.3 mg/m2 dose was determined to be superior to a dose of 1.0 mg/m2.66 Bortezomib is also active as a single agent in earlier stage MM patient populations. A single-agent ORR of 38%, with a 6% complete response (CR) rate, was seen in the phase III APEX study in early relapsed MM, with a time to progression (TTP) of 6.2 months and a median duration of response of 8 months.67 In this study, the major grade 3 and 4 toxicities were PN, 12%; dysesthesia and related symptoms, 8% to 10%; anemia, 8%; diarrhea, 8%; neutropenia, 14%; and fatigue, 12%. In the frontline setting, bortezomib demonstrated a single-agent response rate of 41% (5% CR rate).68
Bortezomib is also approved for newly diagnosed MM in combination with velcade, melphalan and prednisone (VMP). The phase III VISTA trial evaluated VMP in patients with untreated MM who were ineligible for high-dose therapy.69 The addition of bortezomib to the melphalan prednisone (MP) backbone significantly improved response rates in this setting with an ORR of 71% for VMP (including 30% CR) versus 35% (with only 4% CR) for MP.52 VMP was associated with a TTP of ~24 months, compared with ~16.6 months with MP. After a 5-year follow-up, there was a 31% reduced risk of death for the VMP group versus MP-treated patients.70
Bortezomib has also shown promise when combined with other agents in relapsed and refractory MM patients. The combination of bortezomib with pegylated doxorubicin (Doxil, Centocor Ortho Biotech Products, L.P.; Horsham, PA) resulted in an ORR of 79% in relapsed patients, and toxicities were similar to those observed with each agent administered separately.71 A phase III study in 646 patients with relapsed and refractory MM compared this treatment with bortezomib alone; the combination produced a 44% ORR and extended the TTP from 6 to 9.3 months.72,73 The combination of bortezomib with revlimid, lenalidomide and dexamethasone (Rd), a standard of care in the treatment of MM, resulted in an ORR of 64% and a median duration of response of 8.7 months.74 This activity is striking given that 53% of patients had received prior bortezomib and 75% of patients had received prior thalidomide, a closely related analog of lenalidomide. Other agents tested in combination with bortezomib include vorinostat, the anti-CS1 mAb, elotuzumab, the Hsp90 inhibitor tanespimycin, and the Akt inhibitor perifosine.75
Frontline combinations with bortezomib in MM patients have shown high ORRs with a notable improvement in CR rates. In longer term studies, CR rates with bortezomib-based combinations have been shown to be associated with improved clinical outcomes.63,64 A community-based phase IIIb study evaluating bortezomib + dexamethasone (VD) versus bortezomib + thalidomide + dexamethasone (VTD) versus VMP found similar ORR (60%, 70%, and 52%, respectively) and CR rates (13%, 18%, and 15%, respectively).63 Bortezomib + melphalan + prednisone + thalidomide (VMPT) followed by bortezomib + thalidomide (VT) maintenance resulted in a superior CR rate compared with VMP with no maintenance (34% versus 21%) and improved 2-year progression-free survival (70% versus 58.2%).64 A protocol modification in this trial involved changing from twice weekly to weekly bortezomib administration, which yielded similar TTP but reduced the incidence (21% versus 43%) and severity of PN (2% grade 3/4 versus 14%).64 The bortezomib, lenalidomide, and dexamethasone combination in newly diagnosed MM resulted in a ORR of 100% in 66 patients, 29% of whom achieved a CR.76
Bortezomib has also shown activity in other hematologic cancers, most notably mantle cell lymphoma (MCL).77,78 As a single agent in 155 relapsed and refractory MCL patients, bortezomib yielded an ORR of 33% (8% CR), a median duration of response of 9.2 months, and a TTP of 6.2 months.78 Toxicities observed were similar to those seen in patients with MM and included thrombocytopenia, PN, and fatigue. When bortezomib was used to treat both newly diagnosed and refractory MCL, a response rate of 46% was observed in both populations,77 leading to FDA approval late in 2006.
Bortezomib has been tested in a variety of solid tumors in phase I and II studies.79 Partial responses (PR) were reported in 8% of patients with refractory non–small-cell lung cancer (NSCLC), although the TTP was 1.5 months.80 Exacerbation of PN was common. Bortezomib was subsequently tested in combination with paclitaxel, irinotecan, and gemcitabine/carboplatin; however, results have not been encouraging. Bortezomib continues to be tested in combination with other agents in a variety of tumor types.81,82
Recent clinical activity and preclinical data suggest that proteasome inhibition may extend to nononcology applications. Single-agent bortezomib therapy in kidney transplant patients undergoing antibody-mediated rejection resulted in a reduction of donor-specific antibodies and improved renal function.83 In mouse models of lupus nephritis, bortezomib resulted in a reduction of pathogenic plasma cells and the prevention of disease progression.84 These data suggest that PIs may be useful in a wide range of B-cell–mediated diseases. However, toxicities with bortezomib, particularly PN, may prevent wider application of this particular agent.
Carfilzomib
Parallel phase I studies of carfilzomib have been conducted in patients with multiple tumor types, and two phase I dose-finding studies targeting B-cell malignancies have been completed. The first study used daily IV bolus dosing with doses up to 20 mg/m2 for 5 consecutive days followed by 9 days of rest and resulted in substantial inhibition of proteasome activity.85 In the second study, carfilzomib was administered daily for 2 days for 3 consecutive weeks (days 1, 2, 8, 9, 15, and 16), followed by 12 days of recovery.86 Hematologic toxicities were the most frequent adverse events, observed along with transient, noncumulative elevations in serum creatinine, usually with increases in serum urea nitrogen and consistent with a prerenal etiology. New onset PN was infrequent. Among 20 evaluable patients (including bortezomib-refractory patients), 4 PRs and 1 minor response were seen. Responses were also durable, lasting more than 1 year in some cases. Although the maximum tolerated dose of carfilzomib was not established in this study, a dose of 20 mg/m2 was initially selected for the phase II studies.
Based on the phase I studies, an open-label, single-arm, phase II study of single-agent carfilzomib in relapsed and refractory MM was initiated in 2007.87,88 Carfilzomib was administered as an IV bolus on the twice-weekly dose schedule. Patients enrolled in the initial phase of the study (003-A0) had received a median of five prior therapies, and 78% of patients had grade 1/2 PN at entry.87 Among 39 evaluable patients in 003-A0, 10 (26%) achieved a minor response or better, including 5 PRs, and 16 additional patients with stable disease. Based on new safety information from phase I studies, the protocol was amended and the carfilzomib dose was escalated to 27 mg/m2 after the first cycle (003-A1).89 In this trial, 266 patients were enrolled and all patients had previously been treated with an immunomodulatory agent (IMiD) and bortezomib and were refractory to their last therapy. An ORR of 24% with a median duration of response of 8 months was reported. Adverse events were predominantly hematopoietic (thrombocytopenia, lymphopenia, and anemia) and there was a <1% rate of grade 3 PN, despite 77% having a history of PN. Based on these findings, carfilzomib was granted conditional approval by the FDA in 2012 for the treatment of patients with relapsed and refractory myeloma who had received prior bortezomib and IMiD therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree