AJCC 7th Edition (2009)
Note: new staging incorporates Gleason + PSA for risk group determination
Primary tumor:
T1—clinically inapparent tumor neither palpable nor visible by imaging
T1a—incidental histologic finding in 5% or less of tissue resected
T1b—incidental histologic finding in more than 5% of tissue resected
T1c—identified by needle biopsy (e.g., because of elevated PSA )
T2—confined within prostate
T2a—involves one half of one lobe or less
T2b—involves more than one half of one lobe but not both lobes
T2c—involves both lobes
T3—extends through the prostate capsule (note: invasion into the prostatic apex or into, but not beyond, the prostatic capsule is classified not as T3 but as T2)
T3a—extracapsular extension (unilateral or bilateral) or microscopic invasion of bladder neck
T3b—invades seminal vesicles
T4—fixed or invades adjacent structures other than seminal vesicles: bladder, external sphincter, rectum, levator muscles, and/or pelvic wall (note: microscopic invasion of the bladder neck is pT3 and not pT4)
Pathological staging: there is no pT1 classification
Regional lymph nodes:
N0—none
N1—yes
Regional lymph nodes: pelvic, hypogastric, obturator, iliac (internal, external), sacralDistant lymph nodes: aortic, common iliac, inguinal (deep), inguinal (superficial, femoral), supraclavicular, cervical, scalene, retroperitoneal
Distant metastases:
M0—none
M1a—non-regional lymph nodes
M1b—bone
M1c—other sites
Changes from 6th Edition:
New stage groupings incorporate Gleason + PSA
Microscopic bladder neck invasion (previously T4) is now T3a
Table 11.2
NCCN 2010 stage grouping
NCCN stage grouping |
Stage grouping: Note: the AJCC staging manual distinctly lists these as “groups” in contrast to other tumor sites where they are listed as “stage” Group I T1a–c N0 M0, PSA < 10, Gleason ≤ 6 T2a N0 M0, PSA < 10, Gleason ≤ 6 T1–2a N0 M0, PSA X, Gleason X Group IIA T1a–c N0 M0, PSA < 20, Gleason 7 T1a–c N0 M0, PSA ≥ 10 < 20, Gleason ≤ 6 T2a–b N0 M0, PSA < 20, Gleason 7 (corrected in erratum) T2a N0 M0, PSA ≥ 10 < 20, Gleason ≤6 (added in erratum) T2b N0 M0, PSA X, Gleason X Group IIB T2c N0 M0, any PSA , any Gleason T1–2 N0 M0, PSA ≥ 20, any Gleason T1–2 N0 M0, any PSA , Gleason ≥ 8 Group III T3a–b N0 M0, any PSA , any Gleason Group IV T4 N0 M0, any PSA , any Gleason N1, any PSA , any Gleason M1, any PSA , any Gleason |
TNM staging stratification by itself presents a heterogeneous risk grouping several nomograms were statistically generated from outcome data on large groups of men with prostate cancer. They are used to describe to outcome of the disease using well-known diagnostic findings such as pretreatmentPSA , age, Gleason score . Using various predictive factors such as T stage , Gleason score, PSA, and histology results, more accurate assessment of the risk of metastatic spread, lymph node involvement, or recurrence following treatment has been achieved [19–21]. Especially, nomograms may be used by healthcare professionals in partnership with men with prostate cancer to aid decision-making, to help predict biopsy results, to help predict pathological stage, and to help predict risk of treatment failure [2].
To date, modern literature presented more than 20 pretreatment predictive models (probability graphs, nomograms , lookup tables, and neural networks), and mainly all were based on three classical prognostic factors (pretreatment PSA , biopsy-based Gleason score , T stage) [1, 22, 23].
11.3 PSA
PSA is a glycoprotein normally helping to dissolve semen and can be expressed in both benign and malign disorders where PSA leaks out to result an increased level in blood test. The potential benefits of PSA testing in clinic are detection of cancer before symptoms are occurring and/or spreading, diagnosis at early stage, and aid in prostate cancer treatment. The important disadvantage of using PSA as a surrogate marker is that elevated PSA levels may also found in benign prostate hyperplasia, prostatitis, ejaculation and increasing age besides adenocarcinoma [24].
PSA has been used as a useful marker for the diagnosis and follow-up of prostate cancer [24, 25]. The most significant tools for prostate cancer are PSA levels higher than 4 ng/mL and a suspicious digital rectal examination (DRE) even though PSA screening has a limitation of low specificity despite high sensitivity which could easily lead to false-positive diagnoses [25, 26]. The earliest described prognostic factor was pretreatment PSA level which notes that progressively worse biochemical and prostate cancer outcomes were increasing parallel to PSA level [19, 27]. RTOG studies demonstrated that a pretreatment PSA 20 ng/mL predicts a greater likelihood of distant failure [28]. A PSA of 20 ng/mL has been a biochemically alarming sign that was associated with a greater risk of prostate cancer death [18]. Early reports by Partin and colleagues and D’Amico and colleagues defined important PSA cut points of <10, 10.1–20, and >20 ng/mL that are still used to define prostate cancer risk stratification groups [2, 19].
11.4 Gleason Score
Gleason score was firstly described by a pathologist Donald Gleason in 1960 whom he categorized the intrinsic morphologic heterogeneity of prostate cancer into a histological grading system, and since then several studies have clearly established its prognostic value [29, 30]. It has been a standard in urological pathology and could be defined as the most important tool for oncological outcomes. A primary and a secondary pattern (the range of each is 1–5) of the glands defined by microscopy are defined and then summed to a total score. The newly diagnosed needle biopsy-detected prostate cancers are graded Gleason score 6 or above [30]. If a single pattern of disease is seen, it should be reported as both grades. In a radical prostatectomy, if a tertiary pattern is present, it is stated in comment section. Gleason score listing was given in Table 11.3. Recent studies have demonstrated that Gleason score was an extremely important prognostic factor for prostate cancer outcomes [31]. In an analysis of the Radiation Therapy Oncology Group (RTOG) , consisting of more than 1500 men included on prospective randomized trials, Gleason score was found to be the only most important predictor of death from prostate cancer [28, 31]. At the diagnostic pathology, Gleason score has to be defined and taken into consideration for patient personalized treatment selection.
Table 11.3
Gleason scoring system
Score | Definition |
|---|---|
Gleason X | Gleason score cannot be processed |
Gleason 6 | Well differentiated (slight anaplasia) |
Gleason 7 | Moderately differentiated (moderate anaplasia) |
Gleason 8–10 | Poorly differentiated/undifferentiated (marked anaplasia) |
11.5 T Stage
The T stage involves the evaluation of the local extent of the primary tumor in the prostate and its association to adjacent structures. The digital rectal examination (DRE) is still accepted as the “gold standard” for staging which provides almost nonsensitive information for extracapsular extension and depends on the examiner’s experience [32]. Imaging is usually warranted to distinguish between T2 and T3/T4 (spread outside the prostate) cancers where MRI is the commonly used imaging technique for T staging of prostate cancer [33, 34].
AJCC TNM staging system subdivides pT2 disease into three categories pT2a, pT2b, and pT2c as determined by involvement of one half of one side and more than one half of one side and involvement of both sides of the prostate gland. The importance of this subdividing is the representation of the volume of cancer. pT3 disease has been categorized by two sections depending on the presence of extracapsular invasion in any location and presence of seminal vesical invasion with or without extracapsular invasion. Four large retrospective analyses have addressed that microscopic involvement of the bladder neck tissue by prostate cancer does not predict a significantly worse prognosis than extracapsular extension which has been expressed in the new version of staging and revised as pT3a [4].
11.6 Surgical Nomograms
Partin’s table was established as the first nomogram that helps to predict the risk of post-prostatectomy, seminal vesicle invasion, and lymph node positivity after radical prostatectomy as a function of initial PSA , Gleason score, and clinical T stage based on a cohort of 2953 patients [27]. The following important surgical analytical models were published by Kattan and Stephenson (Fig. 11.1 and Table 11.4) [21, 35]. Kattan nomogram relates the classical prognostic factors with biochemical recurrence outcome, whereas the lately stated Stephenson nomogram relates the three classical prognostic factors to 15-year cancer-specific mortality with validation of 82% accuracy in a cohort of 12,677 radical prostatectomy patients [21, 36]. All nomograms described above were surgical nomograms that were developed by urologists.
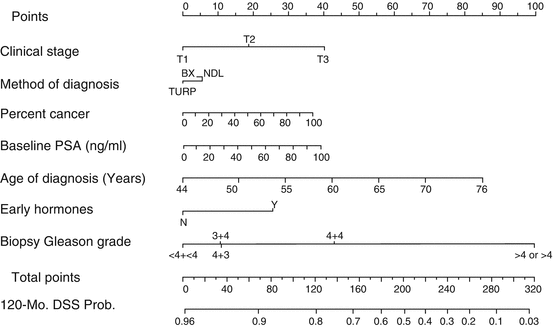

Fig. 11.1
Kattan nomogram . Source: M. Kattan et al., Cancer 112: 69–74, 2008
Table 11.4
Stephenson nomogram : pretreatment risk stratification for prostate cancer
Risk group | Clinical stage | Gleason score | Serum PSA |
|---|---|---|---|
Standard risk groups | |||
Low risk | T1c–T2a | ≤6 | <10 ng/mL |
Intermediate risk | T2b | 7 | 10–20 ng/mL |
High risk | T2c, T3 | 8–10 | >20 ng/mL |
Low risk | ≤T2a | ≤6 | <10 ng/mL |
Intermediate risk | One elevated risk factor | ||
One elevated risk factor: clinical stage ≥T2a disease, Gleason score ≥7, PSA ≥10 ng/mL | |||
High risk | Two elevated risk factors | ||
11.7 Treatment Decision-Derived Nomograms
In 1998, D’Amico and colleagues first suggested a three-group risk stratification system to predict posttreatment biochemical failure after radical prostatectomy or external beam radiotherapy [19]. This system separated nonmetastatic patients into low, intermediate, and high risk grounded on initial PSA , clinical T stage, and biopsy Gleason score . Categories were as follows: low-risk prostate cancer as having 1992 AJCC T1/T2a and PSA ≤ 10 ng/mL and Gleason score ≤ 6, intermediate-risk prostate cancer as having 1992 AJCC T2b and/or PSA 10–20 ng/mL and/or Gleason 7 disease, and high-risk disease as having any one of the following high-risk features—1922 AJCC ≥T2c, PSA >20 ng/mL, or Gleason 8–10 disease [19]. Although this system has been defined for patient selection for radiotherapy, it has been validated in surgical series [37].
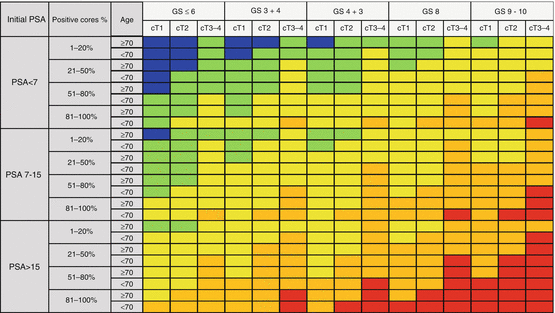
In 2001, The Genito-Urinary Radiation Oncologists of Canada (GUROC) issued the consensus on the prostate cancer topics of risk evaluation, conformal radiotherapy, and brachytherapy and combined hormonal therapy [38]. A consensus of risk stratification for prostate cancer around three categories was defined: (1) low, 1997 AJCC T1–T2a, PSA ≤10 ng/mL, and Gleason ≤6; (2) intermediate, 1997 AJCC T1–T2, PSA ≤20 ng/mL, and Gleason ≤7 not otherwise low risk; and (3) high risk, 1997 AJCC T3–T4 or PSA >20 ng/mL or Gleason 8–10 [38]. In 2013, Rodrigues et al. validated the published three-group risk stratification system in a database that consists of 7974 patients from four Canadian institutions. Additionally, to improve the selection capability, recursive partitioning analysis was developed to determine the sub-stratification of groups defined in 2001 which suggested six separate and statistical unique groups [39]. GUROC low-risk patients were classified as favorable low and low-risk groups based on PSA ≤ 6 and PSA > 6. Additionally GUROC intermediate-risk patients were subclassified into low-intermediate and high-intermediate groups which were intersected by PSA ≥ 10 and either T2b/T2c disease or T1–T2a disease with Gleason 7. An additional extreme-risk group (GUROC high-risk and positive cores ≥ 87.5% or PSA > 30) was added to the GUROC high-risk patient categorization [39]. The GUROC has published a project to revise appropriate definitions of low-intermediate, high-intermediate, and high-risk prostate cancer using a multi-institutional database to using new candidate factors as amount of high-grade cancer, Gleason pattern 3 + 4 versus 4 + 3, percentage of positive biopsy cores, and T stage (i.e., presence of T2b/T2c disease). Available Canadian databases considered for combined analysis will include the British Columbia Cancer Agency prostate cancer database, the National Cancer Institute of Canada PR5 intermediate-risk dose (and dose per fraction) fractionation study, as well as the Princess Margaret Hospital prostate cancer dose escalation and brachytherapy clinical databases [1]. In this project, up to six categories (very low risk, low risk, low-intermediate risk, high-intermediate risk, high risk, and extreme risk) will be defined and characterized in terms of ASTRO BFFS and overall survival. In addition, the ProCaRS database will be used to perform direct propensity score matched-pair analyses of various interventions (e.g., brachytherapy vs. external beam radiation therapy) [1]. Gabriele et al. has published a new classification with a combination of age, pretreatment PSA’ clinical-radiological staging, Gleason score, and percentage of positive cores at biopsy. EUREKA-2 retrospective multicentric database has been used to create “Candiolo” risk classes which are the subgroups of all defined variables and compared to D’Amico staging for progression-free survival and prostate cancer-specific survival and revealed that this system stratifies patients better for these oncological outcomes. Five-year progression-free survival was 94% for very low-risk and 43% for very high-risk group (Fig. 11.2). The major difference from the D’Amico system was the cutoff level of pretreatment PSA level as in this new system defined as 7 ng/mL and 15 ng/mL. Also T2 staging was not divided into subgroups as T2a, T2b, or T2c [40]. External validation studies were warranted to be accepted as a clinical decision-making tool.