Study
Men randomised (n)
Intervention arm (n)
Control arm (n)
Age range (years)
Number of centres
ERSPC
162,243
72,891
89,352
55–69
7
Goteborg
19,904
9952
9952
50–64
1
PLCO
76,685
38,340
38,345
55–74
10
The results of these trials have been published many times since the trials were commenced. The ERSPC and the Goteborg trials show a statistically significant reduction in prostate cancer mortality with a relative risk (RR) of 0.79 (95%, confidence interval (CI) 0.69–0.91) and 0.56 (95%, CI 0.39–0.82) respectively. In contrast, the PLCO trial did not demonstrate a reduction in prostate cancer mortality RR 1.09 (95%, CI 0.87–1.36).
To summarise the potential benefits of screening based on the European studies: For every 1000 men screened there would be 9 fewer deaths (a 28% reduction compared with no screening), 14 fewer men receiving palliative therapy (a 35% reduction as compared to no screening) and a total of 73 life years gained (an average of 8.4 years per prostate cancer death avoided). On the other hand, it brings large numbers of men into the healthcare environment with the possibility of harm caused by both the screening test (biopsy) and the treatment of localised disease.
As there are currently no plans to introduce screening, disease identification is being done on a case finding basis by primary care and point of care PSA testing, alongside digital rectal examination (DRE), once the patient has been given the benefits and risk of PSA screening.
In SSA there are only reports of the results of screening, rather than any controlled trials having been performed. There is data from South Africa (Heyns et al. 2003), where a screening programme was reported based on DRE and/or PSA > 4. It was conducted among 660 men aged 50–70 years of which 60.6% were black. The DRE was reported as suspicious in 3.2% and PSA >4 in 9.6% of men. Only 21 patients were biopsied with a cancer detection rate of 43%, but due to a lack of compliance with the biopsy it is hard to draw any conclusions from this study.
7.3 Presentation and Diagnosis
The major change in the USA and Europe over the past 20 years is the percentage of patients who now present with organ confined disease (80%+). The key challenge for Urologists now is the risk of over-detection of clinically insignificant cancers (there are many definitions, but largely they are cancers with Gleason grade 4, low volume 0.5 ml or less, and PSA below 10), the overtreatment of these cancers and the under-detection of high grade tumours using the standard 10–12 core trans-rectal biopsy technique.
The little data from studies in SSA have shown that there are still a large number of men presenting with advanced/metastatic disease (range 34–62%) (Yamoah et al. 2013; Ekwere et al. 2002). This is due, at least in part, to the lack of awareness of prostate cancer in the general population (Jalloh et al. 2008; Ajape et al. 2009) There is also a significant lack on a population basis of trained Urologists. The optimal diagnostic technique would involve low morbidity and cost, and be able to reliably identify cancers likely to cause harm to the patient.
7.4 Diagnosis
7.4.1 Prostate Biopsies
The mainstay of diagnosis in the USA and Europe for the past 20 years has been trans-rectal prostate biopsy carried out under local anaesthetic using a rectal ultrasound probe to guide the biopsies into 12 distinct areas of the peripheral zone of the prostate (Fig. 7.1). The ability of the ultrasound to detect abnormal lesions within the prostate is dependent on both the technology being available to deliver a sharp image and also the operator having the experience to recognise and target the abnormality (Toi et al. 2007).
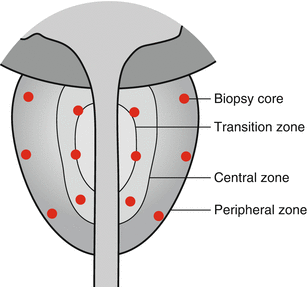

Fig. 7.1
Standard 12 core protocol
The European Association of Urology (EAU) guidelines for trans-rectal biopsy are:
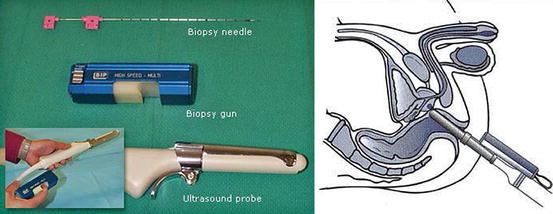
It should be performed under ultrasound guidance (Fig. 7.2)
It should be carried out using local anaesthetic periprostatic block
It should be carried out under antibiotic cover (intravenous gentamicin and ciprofloxacin or variations) depending on local antibiotic resistance
An extended 10–12 core biopsy protocol should be used

Fig. 7.2
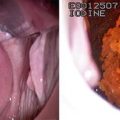
Transrectal ultrasound scan (USS) probe (left) and technique (right)
7.4.2 Indications for Biopsies
There are currently two indications for a prostate biopsy: an abnormal DRE and a raised PSA. Since the use of PSA testing began in the 1980s there has been a gradual lowering of the threshold of PSA that triggers a biopsy. In the ERSPC, the threshold was 3 ng/ml, but age related ranges and higher values are also in widespread usage. The risk of prostate cancer diagnosis rises with the PSA level, but the Prostate Cancer Prevention Trial (PCPT) demonstrated that there is no safe lower level of PSA at which the risk of high grade disease is zero (Thompson et al. 2004). It is considered good practice to repeat any mildly raised PSA before proceeding to a biopsy.
7.4.3 Biomarkers and the Beckman Coulter Prostate Health Index
There has been interest in reducing the number of unnecessary biopsies undertaken without lowering the detection of high grade tumours. Biomarkers have been used to improve the diagnostic accuracy of PSA. They include: the ratio of prostate cancer gene 3 (PCA3) ribonucleic acid (RNA) to PSA RNA, serum PSA parameters (PSA velocity, PSA doubling time, percentage free PSA, PSA density, and age-specific PSA), and panels of serum kallikreins (total PSA, free PSA and kallikrein-related peptidase 2). These have been used, or are currently being evaluated, to try to reduce the number of unnecessary biopsies (Bryant et al. 2014). The Beckman Coulter Prostate Health Index (PHI) uses a mathematical formula combining total PSA, percentage free PSA and pro-PSA and seems to be a significant predictor of prostate cancer at initial prostate biopsy in men whose PSA is between 2 ng/ml and 10 ng/ml (Guazzoni et al. 2011).
The decision to recommend a prostate biopsy is an individual one and will depend on some of the factors mentioned above, and also the family history and general health of the patient. It should be noted, however, that there is currently an on-going recent move away from trans-rectal biopsies due to increasing problems with antibiotic resistance making infection post-biopsy more likely. The concern over the both the over-diagnosis of biologically insignificant tumours and post-biopsy sepsis has led to an increased use of pre-biopsy imaging with MRI scanning.
7.4.4 Digital Rectal Examination
A DRE has a poor sensitivity and specificity, but if abnormal, is an indication to perform a prostate biopsy irrespective of the PSA result – as an abnormal DRE is usually indicative of a cancer with a higher Gleason score (Okotie et al. 2007). Although the data is poor from SSA, the studies that have been undertaken have shown a high percentage of abnormal DRE and high clinical stage (stage > T2) from 20.2% in Yamoah et al.’s (2013) study in Ghana to 81.4% in Ekwere et al.’s (2002) Nigerian patients. This is as one would expect due to the low use of PSA testing and influences the likely treatment options available to the majority of these patients.
7.4.5 The Role of Pre-biopsy Magnetic Resonance Imaging
Multi-parametric magnetic resonance imaging (mpMRI) can potentially visualise prostate cancer tumours larger than 0.5 cm3 with 93% sensitivity and 98% negative predictive value (Pokorny et al. 2014), but there is controversy surrounding its use as a sole diagnostic tool as recent reports suggest it may miss up to 26% of significant tumours found in removed prostates (Le et al. 2014). The reporting of mpMRI will improve and although not currently the standard of care this situation is likely to change due to the impetus to reduce the unnecessary number of biopsies being performed and to reduce the post biopsy sepsis rate. The mpMRI approach allows a targeted biopsy performed under a general anaesthetic significantly reducing the sepsis rate from 2 to 3% for the transrectal approach to a more acceptable 0.1%. It is also becoming the recommended approach to monitor those patients on active surveillance for low-risk prostate cancer (Fig. 7.3).
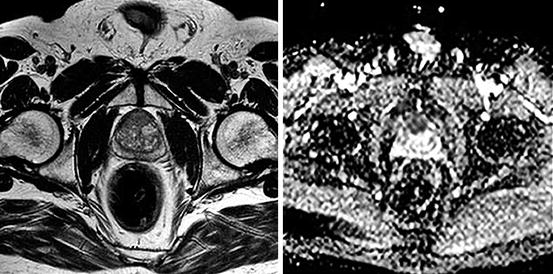

Fig. 7.3
PI-RAD five anterior prostate cancer on MRI. T2 (left) and diffusion (right) weighted images
7.5 The Management of Patients with an Initial Negative Biopsy
The standard approach in the United Kingdom (UK) has changed with the introduction of new guidance from the National Institute of Clinical Excellence (NICE; the UK’s “ independent organisation responsible for developing national guidance, standards and information on providing high-quality health and social care, and preventing and treating ill health”) which now recommends mpMRI in patients with an initial negative prostate biopsy and in whom there is still concern with regard to a diagnosis of prostate cancer (rising PSA, abnormal DRE; (Excellence 2015)). This is often used in conjunction with the template trans-perineal biopsy technique to target the abnormal lesion. It is recognised that most men with a negative biopsy and a negative mpMRI are likely at most to have a low grade, low volume and therefore low risk prostate cancer, with less than 5% having intermediate or high risk disease (Le Maitre et al. 2009).
7.5.1 Pathological Diagnosis of Prostate Cancer in SSA
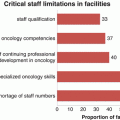
A recent study audited the prostate cancer biopsy practice in centres of six SSA countries (Senegal, Ghana, Sudan, Uganda, South Africa and Botswana) (Jalloh et al. 2013). In total, only 4672 black men underwent prostate biopsy between the years of 2005 and 2011. Of these 1241 (27%) had a positive biopsy with the highest Gleason grades (Schroder et al. 2014; Hugosson et al. 2015; Andriole et al. 2012) in Sudan and Uganda. There was criticism of the biopsy techniques used in these centres and none complied with the current EAU guidelines as mentioned above. One of the major problems encountered in these countries is the lack of pathologists. A survey report by showed that in 2012 the highest number of pathologists per person was 1:226,470 in Botswana and 1:297,000 in SA followed by 1:500,000–1,000,000 in Ghana Kenya and Nigeria with all the other SSA countries having a greater ratio than 1:1,000,000. In comparison the ratios in the UK is 1:15,000 with a large proportion having a sub-speciality interest.
7.5.2 Treatment of Prostate Cancer
The treatment of prostate cancer is dependent upon the stage and grade of the cancer at presentation, and the general fitness of the patient. There has been a trend over the last 10 years to offer active surveillance for patients with low risk disease. There are many risk categories based on the PSA, cancer grade and the number of cores affected, as well as the clinical stage on DRE.
The most commonly used is the D’Amico category (D’Amico et al. 2005):
Low Risk:
PSA < 10 ng/ml and Gleason <6 and percentage of involved cores is <50%
or
Intermediate risk with only 1 positive core
Intermediate Risk:




Gleason score of 7
or
PSA of 10–20
or
Low risk with >50% of positive cores
or
High risk and only 1 positive core
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree