Citation
Study population
Survival data, selected prognostic factors, comments
Altman and Cadman (1986) [30]
1539 cases from a single center (USA), 1922–1981
Median OS 5 months. 56 % of cases received therapy, 18 % were not confirmed histologically. Histologically confirmed cases that received therapy showed a median OS of 7 months
Alberts et al. (1989) [31]
100 cases from a single center (South Africa), 1977–1984
Median OS 124 days. RF: performance status
Pavlidis et al. (1994) [32]
85 cases from three centers (Greece), 1986–1991
Aim was to study serum tumor markers. Median OS not specified, was about 5 months as indicated by figure. CA 19–9 and CA 15–3 levels correlated with number of metastases
Abbruzzese et al. (1994) [33]
657 cases from a single center (USA), 1987–1992
Median OS 11 months. An extended study of this population was published later once more [34]; see next row
Hess et al. (1999) [34]
1000 cases from a single center (USA), 1987–1994
Median OS 11 months. Two prognostic models with ten and nine groups, respectively. RF: number of metastatic sites, adenocarcinoma, liver and adrenal metastases. Protective factor: neuroendocrine carcinoma
Van de Wouw et al. (2002) [35]
1285 cases from a cancer registry (Netherlands, representing about one million inhabitants), 1984–1992
1024 cases biopsy proven, 261 only clinically diagnosed. Biopsy-proven cases: median OS 11 weeks, only supportive therapy in 67 %. Protective factors: age <50 years, lymph node metastases
Levi et al. (2002) [1]
699 cases from a cancer registry (Switzerland, representing 786.000 inhabitants), 1984–1993
543 biopsy-proven cases: median age 71 years, median OS 11 weeks. 156 only clinically diagnosed cases: median age 79 years, median OS 6 weeks. Protective factor: squamous cell carcinoma (median OS 41 weeks)
Culine et al. (2002) [36]
150 cases from a single center (France), 1989–1999
Median OS 7.5 months. RF: performance status, liver metastases, LDH
Van de Wouw et al. (2004) [37]
70 cases from a single center (Netherlands), 1990–1996
Median OS 12 weeks. RF: age ≥60 years, performance status, number of metastatic sites, liver metastases, LDH
Seve et al. (2006) [38]
317 cases from a single center (Canada), 1998–2004
Median OS 104 days. RF: liver metastases, low serum albumin, performance status
Ponce Lorenzo et al. (2007) [39]
100 cases from a single center (Spain), 2002–2006
Median OS 4.7 months. RF: performance status, number of metastatic sites, liver metastases
Trivanovic et al. (2009) [40]
145 cases from two centers (Croatia), 2002–2007
Median OS 330 days. RF: performance status, liver metastases, LDH, anemia, age, prolonged QT interval
Thöm et al. (2009) [41]
136 cases from a single center (Germany), 1989–1998
Median OS 7.9 months. RF: performance status, male sex
Fernandez-Cotarelo et al. (2010) [42]
265 cases from a single center (Spain),1999–2003
Median OS 2.5 months. RF: age, AP, low albumin. Protective factor: squamous cell carcinoma
Petrakis et al. (2013) [43]
311 cases from a single center (Greece), 1988–2011
Median OS 8 months. RF: performance status, leukocytosis, visceral metastases
Löffler et al. (2014) [2]
223 cases from a single center (Germany), 2006–2010
Median OS 16.5 months. RF: performance status, number of metastatic sites, liver and adrenal metastases
It should be noted that these case series are the most valid evidence available regarding overall survival of unselected CUP patients. Nevertheless, reported overall survival varied between 11 weeks and 16.5 months in these publications. A closer look reveals that the worst overall survival of 11 weeks was reported by two epidemiological registries, while the best outcomes were reported by specialized oncologic centers (Table 4.1). This seems to indicate that patient selection may partly explain these discrepancies since there may be a proportion of frail or terminally ill patients that never reach a specialized oncologic center, as discussed in detail in Sect. 2.2 of this book. On the other hand, there seems to be a trend that better survival and higher rates of patients treated with cytostatics in the newer as compared to the older series reflect some improvement in therapeutic options. Prognostic factors might be useful to overcome these selection effects and predict survival of individual patients better than pooled data of heterogeneous patient cohorts.
4.4 Specific Prognostic Factors
Given the small number of publications addressing prognostic factors in CUP patients, the number of reported prognostic factors is rather high. However, when only parameters with prognostic significance confirmed by multivariate analysis in several independent investigations are considered, fewer parameters remain. Moreover, the practical utility of a given prognostic parameter is influenced by additional factors, e.g., the question whether this information is generally available for all patients or requires special tests and the related question whether this information can be determined in an unequivocal, objective, easy-to-handle fashion, which might be problematic for some parameters such as performance status or comorbidity. With the following list, we focus on the most relevant and best-established prognostic factors in CUP patients.
Age: Several case series found higher age to be correlated with poorer outcome [17, 34, 37, 42, 44]. For example, Van de Wouw et al. [37] reported a median overall survival of 30 weeks in 17 patients aged less than 60 years, whereas median survival was 12 weeks in 53 patients aged 60 years or older (p = 0.016). On the other hand, several other series including our own collection of 223 cases failed to find any correlation between age and prognosis [2, 33]. For example, the large series of Abbruzzese et al. [33] reported median survival times ranging from 8.7 months in patients aged 20–39 years to 6.4 months in the age group of 70 years and older, with widely overlapping confidence intervals for all age groups. Apparently, age as a prognostic factor seems to be particularly sensitive to treatment decisions since the influence of age on the decision between aggressive therapy and best supportive care might differ between institutions and might have changed over time. In conclusion, we do not think that there is evidence that age is a useful prognosis indicator.
Sex: There is good evidence from several publications that male sex is associated with poorer outcome than female sex [2, 31, 33, 41]. Abbruzzese et al. [33] reported median survivals of 9.6 months in women and 6.4 months in men, and the corresponding data from our own series were 19.3 months in female versus 12.3 months in male patients [2]. It seems likely that these differences mainly reflect different distributions of sites of origin since gynecological primaries are associated with more favorable outcome, while the prognostically dismal group of smoking-related cancers is probably more frequent in men. Whether additional, more general sex-specific differences in tumor biology exist, remains an open question.
ECOG/WHO performance status: The performance status according to Eastern Cooperative Oncology group [45], describing cancer-associated reduction of physical activity and also referred to as WHO, Zubrod, or ECOG score, is among the best-established prognosis predictors for CUP patients [2, 31, 36–41, 43, 46–48]. While survival seems to worsen with each step the performance status declines, patients are commonly grouped into a favorable group with a performance status of 0–1, and an unfavorable group with a performance status >1, with the latter showing survival times less than half of that of the reference group.
Number of metastatic sites: To quantify metastatic dissemination, the number of involved organs sites (e.g., lymph nodes, liver, lungs) may be counted. Several publications found a correlation of this number with survival [2, 33, 34, 37, 41]. Dichotomization of prognosis groups may include patients with just one or up to two involved organ sites in the favorable group.
Specific sites of metastasis: Not surprisingly, specific organ sites affected by metastases show associations with survival, and many such associations have been published. The best-established predictor of poor outcome is liver metastases, which showed significant correlations with worse survival in several independent investigations [2, 31, 33, 34, 37–40]. Adrenal metastases might be an indicator of remarkably poor prognosis, with survival being less than half of the reference group when they are present, as reported in two independent studies, although it should be mentioned that these data are based on a small number of cases [2, 34]. In our own series of 223 cases, the hazard ratio for ten patients with adrenal metastases was 2.7 (1.1–6.2), but the significance of adrenal metastases as prognostic parameter was lost after correction for confounders, especially since the overall number of metastatic organ sites was higher in this patient group [2]. Finally, some authors have reported affection of lymph nodes as being correlated with favorable outcome [33, 41], while other cohorts did not reveal this correlation, although the majority of studies showed a trend toward better survival with lymph node metastases [2, 31, 37–40], allowing the conclusion that there may be a moderate positive impact of lymph node affection on survival.
Specific histologic groups: When compared to other histologic categories as reference, both squamous cell and neuroendocrine carcinomas have been reported as prognostically favorable, while adenocarcinomas – the majority of CUPs – are associated with a poorer prognosis [1, 33, 34, 42, 43]. The latter seems to result from of the fact that selecting adenocarcinomas implies a selection against most prognostically favorable subgroups. Selection of favorable subsets may also be the reason why squamous cell carcinomas are prognostically favorable since locally restricted cancers are more frequent in this histologic group. Evidence regarding the question whether squamous cell carcinomas are associated with favorable outcome also in disseminated stages is limited. For example, in the large collection of Abbruzzese et al. [33], the prognostic relevance of adenocarcinoma (conferring poor outcome) and neuroendocrine carcinoma (conferring favorable outcome) remained significant, while squamous cell histology showed no significant correlation with outcome in multivariate analysis.
Serum tumor markers: Few studies exist that address the prognostic or predictive value of serum tumor markers in CUP. There is some evidence that the extent of serum tumor marker elevation in general is correlated with disease stage, and markers elevated above a threshold therefore indicate poor survival, which is expected due to the general correlation of tumor markers with tumor mass [2, 32, 43]. On the other hand, no specific tumor marker that, in comparison to other tumor markers, shows a qualitative correlation with outcome of CUP patients has been described so far. One obvious limitation of the studies published up to now is that serum tumor markers were not prospectively evaluated in all patients included, so each tumor marker was available only in a subset of the respective study population.
Other blood tests: Serum lactate dehydrogenase (LDH), alkaline phosphatase (AP), and albumin levels have each been verified as prognostic parameters by several independent investigations [36–40, 42, 46, 49]. Each of these parameters, when elevated above the upper normal limit (LDH, AP) or reduced below the lower limit of normal (albumin), respectively, is associated with an overall survival less than half of that of the reference group with normal levels. Taking into account that abnormalities of these parameters affect only a minority of patients, it seems that they nonspecifically indicate an advanced disease stage. The same probably applies to other blood tests whose role in the prognostication of CUP has been less well characterized: An association with poor prognosis was also reported for leukocytosis, anemia, and lymphopenia [38, 43].
Other parameters: Various prognostic factors not mentioned so far have been reported. One study found a strong association of a prolonged QT interval corrected for heart rate (QTc interval) with poor outcome [40]. An obvious disadvantage of this parameter (and probably the reason why there is only one publication addressing it) is that it is not widely assessed in routine care of CUP patients. The same probably applies to the Adult Comorbidity Evaluation 27 (ACE-27) index, a 27-item comorbidity index designed for cancer patients: Seve et al. [38] found a strong correlation of this index with outcome that remained significant in multivariate analysis, thereby being an independent prognostic parameter in addition to ECOG performance status, number of metastatic sites, and other parameters. Although application of the ACE-27 index therefore appears generally desirable and will be useful in scientific settings, we dare to predict that its establishment in routine practice will be difficult, but nevertheless oncologists should keep in mind that comorbidities are among the major factors affecting outcome of CUP patients, as it is the case for cancer in general.
Finally, it should be mentioned that some of the cited case series also analyzed the impact of therapy on prognosis. As expected, patients treated with chemotherapy or other tumor-specific therapies survived longer than patients who did not receive any tumor-specific treatment, and patients treated with surgery and/or radiotherapy survived longer than patients treated with chemotherapy, which probably reflects selection of patients suitable for these treatment modalities because of locally confined disease [2, 41, 42, 48, 49].
4.5 Prognostic Scoring Systems
Keeping in mind the above list of prognostic factors, it appears desirable to define a scoring system, ideally integrating all prognostic information into one single readout. In the following section, we will discuss, in chronological order, five different scoring systems that have been established for CUP patients. Beforehand, it should be mentioned that eligibility criteria for the respective patient cohorts varied in details between the studies discussed, some of which excluded prognostically favorable subsets by using various definitions. Nevertheless, we think that all scoring systems discussed below are generally applicable to CUP patients.
Based on regression tree analysis of 1000 consecutive CUP patients, Hess et al. [34] developed two different trees with ten and nine terminal subgroups, respectively. The regression trees incorporated different sites of involvement (liver, bones, adrenals, pleura, lymph nodes), number of metastatic sites (≤2 versus >2), histology (with neuroendocrine carcinomas as favorable group and adenocarcinomas as unfavorable group), and age (with 61.5 years as cutoff) as separate prognostic parameters. Median survival in the terminal subgroups varied between 45 and 5 months, although many groups showed overlapping confidence intervals and there was no validation cohort. We conclude that this study is a valuable resource for understanding of prognostic factors and their relative weight for the prognostication of CUP patients, but with respect to the complicated algorithms and lack of external validation, the proposed classification does not appear suitable for clinical application on a broader basis.
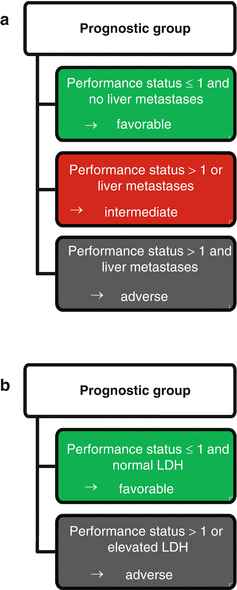
In contrast, Culine et al. [36] aimed to establish a simple and broadly applicable prognostic model. Considering only clinical variables in a cohort of 150 unselected CUP patients, they defined three groups (Fig. 4.1a): The favorable group, defined by a performance status of ≤1 and no liver metastases, showed a 1-year survival of 43 % and a median overall survival of 10.8 months. In the intermediate group with a performance status >1 or liver metastases, the 1-year survival was 23 % and the median survival 6.3 months. The prognostically poor group with both a performance status >1 and liver metastases displayed a 1-year survival of 0 % and a median overall survival of 2.4 months. With the addition of serum LDH levels (which were available in 81 out of the 150 patients), the independent prognostic significance of liver metastases was lost. The authors therefore defined a second prognostic model with only two groups, a favorable group with a performance status of ≤1 and normal LDH (or absent liver metastasis if LDH was unknown) and an unfavorable group comprising all other cases (Fig. 4.1b). The 1-year survivals were 45 % in the favorable versus 11 % in the unfavorable group, and the respective overall survival times were 11.7 and 3.9 months. This second model was verified in an external validation cohort generated from two prospective phase II trials, which showed 1-year survival rates of 53 % versus 23 % and median overall survivals of 12 versus 7 months (p = 0.0089). We conclude that this prognostic model stands out due to independent validation and feasibility in routine patient care. Possible criticism may include that the presence of only two groups and the omission of several parameters known to be prognostically relevant only allows for crude classification, and that in 46 % of the study population, LDH was substituted by liver metastases as prognostic parameter, leaving open which of these parameters should be preferred in clinical practice.