A. Primary tumor (T)
TX
Primary tumor cannot be assessed
T0
No evidence of primary tumor
Tis
Carcinoma in situ
Ta
Noninvasive verrucous carcinoma
T1a
Tumor invades subepithelial connective tissue without lymph vascular invasion and is not poorly differentiated (i.e., grades 3–4)
T1b
Tumor invades subepithelial connective tissue with lymph vascular invasion or is poorly differentiated
T2
Tumor invades corpus spongiosum or cavernosum
T3
Tumor invades urethra
T4
Tumor invades other adjacent structures
B. Regional lymph nodes (N)
Clinical stage definition
cNX
Regional lymph nodes cannot be assessed
cN0
No palpable or visibly enlarged inguinal lymph nodes
cN1
Palpable mobile unilateral inguinal lymph node
cN2
Palpable mobile multiple or bilateral inguinal lymph nodes
cN3
Palpable fixed inguinal nodal mass or pelvic lymphadenopathy unilateral or bilateral
Pathological stage definition
pNX
Regional lymph nodes cannot be assessed
pN0
No regional lymph node metastasis
pN1
Metastasis in a single inguinal lymph node
pN2
Metastases in multiple or bilateral inguinal lymph nodes
pN3
Extranodal extension of lymph node metastasis or pelvic lymph node(s) unilateral or bilateral
C. Distant metastasis (M)
M0
No distant metastasis
M1
Distant metastasisa
Table 5.2
Anatomic stage/prognostic groups
Stage | T | N | M |
|---|---|---|---|
0 | Tis | N0 | M0 |
Ta | N0 | M0 | |
I | T1a | N0 | M0 |
II | T1b | N0 | M0 |
T2 | N0 | M0 | |
T3 | N0 | M0 | |
IIIa | T1–3 | N1 | M0 |
IIIb | T1–3 | N2 | M0 |
IV | T4 | Any N | M0 |
Any T | N3 | M0 | |
Any T | Any N | M1 |
Predicting Positive Inguinal Lymph Nodes
Histological Grade
Tumor grade has long been recognized as providing added value to stage in patients with squamous penile cancer. In 1989, Fraley et al. were one of the earliest investigators who identified a relationship between stage and histological differentiation in the predicting of metastases to the inguinal lymph nodes [2]. They reported that of the 23 cases of carcinoma in situ or well-differentiated disease, only 1 became metastatic, while of the 35 cases of moderately to poorly differentiated disease, 31 metastasized to the groin. Similar data were reported by McDougal in a series of 76 cases, where 82.7 % of the patients with poorly differentiated or invasive tumors had lymph node metastases [3]. As described by a number of studies detailed below, grade often serves as a significant parameter on multivariate analysis of predictors for lymph node metastases.
Recently, Chaux et al. found that comparison of tumor at the time of the primary diagnosis and of recurrence shows that histological subtype and grade were identical in 76 % of the cases and converted to a higher-grade tumor in 24 % of the cases [4]. Eighty percent of patients with high-grade tumors died from penile cancer. In addition, Chaux et al. developed a grading model for penile cancer to determine the influence on nodal metastasis of the various proportions of grades. When histologically evaluating penile carcinomas, they recommended a careful search of areas of grade 3. They concluded that any focus of grade 3 should be sufficient to grade the neoplasm as a high-grade tumor in order to enhance predictability of nodal metastases [5].
Lymphatic Invasion
In a cancer where there is orderly metastasis along lymphatic chains, invasion of the lymphatic system in the primary tumor might be expected to be a strong prognostic factor. Lopes et al. studied prognostic factors in 145 patients with penile cancer treated with penile amputation and lymphadenectomy [6]. The 5-year disease-free and overall survival rates were 45.3 and 54.3 %, respectively. Venous and lymphatic embolizations were the main factors significantly affecting the incidence of lymph node metastasis, which were the main risk factors for recurrence and death, while level of invasion into adjacent structures was not a significant predictor.
Slaton et al. reported on 48 patients treated at the MD Anderson Cancer Center [7]. They found that none of 15 pT1 tumors exhibited lymphovascular invasion or lymph node metastases. Of 33 patients with pT2 or greater tumors, 21 (64 %) had lymphovascular invasion and 18 (55 %) had metastases. Only 4 of 25 patients (15 %) with 50 % or less poorly differentiated cancer in the penile tumor had metastases compared with 14 of 23 patients (61 %) with greater than 50 % poorly differentiated cancer (p = 0.001).
When Ficarra et al. analyzed a number of pathological features, they found that only venous embolization, lymphatic embolization, pT stage, and histological grade were independent predictors of lymph node metastases [8]. In addition, in a subgroup analysis performed in cN0 patients, vascular and lymphatic embolizations were shown to be the most powerful predictors of nodal metastasis (p = 0.01).
Combining Factors for Risk Group Formation
As noted above, investigators are making efforts to combine various prognostic factors to predict nodal metastases. Solsona et al. first reported an attempt to formally combine stage and grade in an effort to stratify patients by risk factors. In their analysis, patients with pT1G1 disease could be classified as having a low risk of nodal involvement, while those with pT1G2–3 or pT2G1 as having an intermediate risk, and those with pT2 grades 2–3 or greater than stage pT3 as having a high risk [9]. The percentage of nodal metastases in the low-, intermediate-, and high-risk groups were 0, 36.4, and 80 %, respectively. This classification was confirmed in 2001 in 37 patients in whom the percentages of inguinal metastases were 0 % for the low-, 33 % for the intermediate-, and 83 % for the high-risk group [10].
The European Association of Urology (EAU) guidelines suggest a different classification: (a) low risk for those with stage pTis, pTaG1–2, or pT1G1 disease; (b) intermediate risk for those with pT1G2 tumors; and (c) high risk for those with stage pT2 or greater or G3 cancer [11]. The risk of inguinal metastases according to the EAU classification was 4 % for low-risk, 34.8 % for intermediate-risk, and 45.8 % for high-risk patients. Unfortunately, Novara et al. showed that both the Solsona et al. and the EAU risk groups had low prognostic accuracy [12, 13]. These efforts to incorporate grade and lymphovascular embolization laid the groundwork for subdivision of T1 into T1a and T1b in the updated AJCC guidelines.
Generation of Nomograms
Nomograms represent the most contemporary effort to stratify patients by risk factors [14]. Chaux et al. proposed the prognostic index to predict for metastasis of penile cancer including multiple factors associated with depth of invasion (in glans, lamina propria, numerical value of 1; corpus pongiosum, 2; and corpus cavernosum). In foreskin, they were lamina propria, numerical value of 1; dartos, 2; and skin, 3 [15]. A logistic regression model was the basis for the nomogram. The nomogram was validated by (a) calculation of the concordance index to evaluate discrimination and (b) assessment of calibration, grouping patients with respect to their nomogram-predicted probabilities and then comparing the mean of the group with the observed proportion of positive lymph nodes.
Mean follow-up obtained in all patients was of 81 months. The distribution of cases and rate of metastasis according to index scores were 2 (1 case), no metastasis; 3 (17 cases), no metastasis; 4 (35 cases), 20 % of metastasis; 5 (50 cases), 50 % of metastasis; 6 (47 cases), 66 % of metastasis; and 7 (43 cases), 79 % of metastasis. On logistic regression analysis evaluating various pathological factors, prognostic index scores were found as the best predictors of inguinal node metastasis and patients’ survival compared to stage and grade.
Ficarra and Kattan developed a formal nomogram to predict nodal involvement [16]. They collected the clinical and pathological data of 175 patients who had undergone surgical therapy for squamous cell carcinoma of the penis from 1980 to 2002 at 11 urological centers in northeastern Italy. A logistic regression model was used to construct the nomogram. Data was integrating from eight clinical and pathological variables (i.e., clinical inguinal lymph node stage, pathological tumor thickness, growth pattern, histological grade, lymphatic and/or venous embolization, corpora cavernosa infiltration, corpus spongiosum, and/or urethral infiltration; Fig. 5.1). The nomogram had excellent concordance index (0.876). However, this nomogram has yet to undergo external validation.
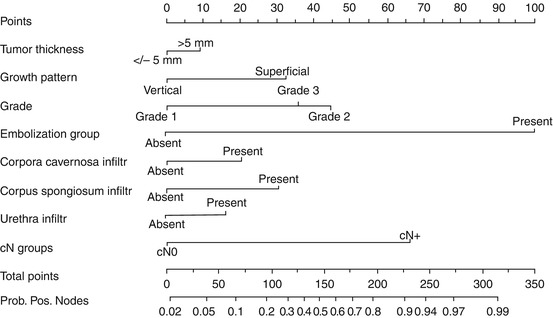

Fig. 5.1
Nomogram predicting probability of pathological lymph node involvement. Probability positive nodes, probability of pathologically positive lymph node. See Appendix I for instructions on use (Reprinted from Ficarra et al. [16], Copyright 2006, with permission from Elsevier)
More recently, Zhu et al. report on a group of 110 men with penile cancer and clinically negative lymph nodes from 1990 to 2008 [17]. The final model, presented as a nomogram, included T stage, grade, lymphovascular invasion, and p53 expression (Fig. 5.2). Only lymphovascular invasion showed independent prognostic value on multivariate analysis (p = 0.024). The model also showed good calibration (bootstrap-corrected concordance index 0.79). The primary difference between the Kattan and Zhu nomogram was the inclusion of the biomarker, tumor suppression protein p53.
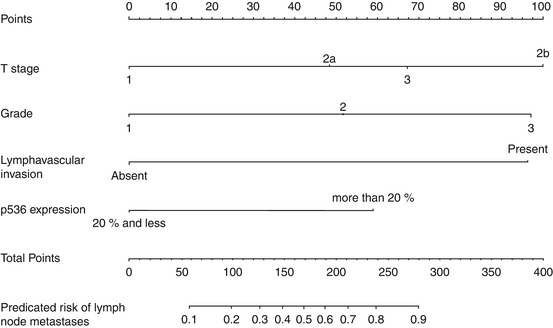

Fig. 5.2
Nomogram predicting lymph node involvement (Reprinted from Zhu et al. [17], with permission from Elsevier)
Predicting Cancer-Specific Survival
It has been a challenge to study the prognostic factors for cancer-specific survival in patients with penile carcinoma due to the limited number of patients included in published series.
N Stage
A number of investigators have reported on the value of TNM N stage for predicting survival after lymph node dissection (Table 5.3) [18–29]. For N0 patients, 3–5-year survival ranges from 85 to 100 %, for N1 79 to 100 %, for N2 7 to 73 %, and for N3 0 to 67 %. Zhu et al. studied how changes in the N stage from the 6th edition of the AJCC staging system to the 7th edition impacted survival [28]. In the 7th edition, there is no longer a staging distinction between inguinal superficial and deep lymph nodes, and extranodal extension of regional lymph node metastases is classified as N3 disease. Among a group of 60 patients, using the 6th edition N classification, the 3-year recurrence-free survival rate was 69.8, 48.2, and 33.3 % for the N1, N2, and N3 categories, respectively. However, log rank survival analysis failed to show a statistical difference (p = 0.054). In the new 7th edition N categories, the 3-year recurrence-free survival rate was 87.5, 57, and 31.8 % in the corresponding N1 to N3 groups. Better survival stratification was observed on analysis (p < 0.001). Thus, the new N staging system better reflects the prognosis in patients with penile cancer.
Table 5.3




Five-year cancer-specific survival stratified by pathological nodal stage
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree