The clinical heterogeneity of myelodysplastic syndromes (MDS) is best illustrated by the observation that these disorders range from indolent conditions with a near-normal life expectancy to forms approaching acute myeloid leukemia (AML). A risk-adapted treatment strategy is mandatory for conditions showing a highly variable clinical course, and definition of the individual risk has been based so far on the use of prognostic scoring systems. The authors have developed a prognostic model that accounts for the World Health Organization (WHO) categories, cytogenetics, transfusion dependency, and bone marrow fibrosis. This WHO classification-based Prognostic Scoring System (WPSS) is able to classify patients into five risk groups showing different survivals and probabilities of leukemic evolution. WPSS predicts survival and leukemia progression at any time during follow-up, and may therefore be used for implementing risk-adapted treatment strategies.
Myelodysplastic syndromes (MDS) are clonal hematopoietic stem cell disorders characterized by ineffective hematopoiesis, peripheral cytopenias, and substantial risk for progression to acute myeloid leukemia (AML). They typically occur in elderly people with a median age at diagnosis ranging between 65 and 75 years in most series, and the annual incidence exceeds 20 per 100,000 persons more than 70 years of age.
Myelodysplastic syndromes were defined and classified in 1982 by the French American British group. In 2001 the World Health Organization (WHO) classification of myeloid neoplasms was developed ; this classification has been updated in 2008. The current diagnostic approach includes peripheral blood and bone marrow morphology (to evaluate abnormalities of peripheral blood cells and hematopoietic precursors); bone marrow biopsy (to assess marrow cellularity, fibrosis and topography); and cytogenetics (to identify nonrandom chromosomal abnormalities). The combination of overt marrow dysplasia and clonal cytogenetic abnormality allows a conclusive diagnosis of MDS, but this is found in only a portion of patients. Although bone marrow biopsy may be considered too invasive for elderly patients, it provides extremely useful diagnostic and prognostic information regarding cellularity, fibrosis, and CD34-positive cell topography.
Prognostic factors in myelodysplastic syndromes
The clinical heterogeneity of myelodysplastic syndromes is best illustrated by the observation that these disorders range from indolent conditions with a near-normal life expectancy to forms approaching AML. A risk-adapted treatment strategy is mandatory for conditions showing a highly variable clinical course, and definition of the individual risk has been based so far on the use of prognostic scoring systems.
In 1997, Greenberg and coworkers developed the International Prognostic Scoring System (IPSS), based on bone marrow blasts, cytogenetic abnormalities, and number of cytopenias ( Table 1 ). The IPSS was derived from a multivariate analysis of hematological characteristics of 816 subjects at clinical onset, also including subjects with 20% to 30% marrow blasts, now considered as having AML. Despite these limitations, IPSS proved to be useful for predicting survival and acute leukemic risk in individuals with MDS and has been incorporated to the design and analysis of therapeutic trials in these disorders.
| Variable | Variable Scores | |||||||
|---|---|---|---|---|---|---|---|---|
| 0.0 | 0.5 | 1.0 | 1.5 | 2.0 | ||||
| Bone marrow blasts (%) | <5 | 5–10 | — | 11–20 | 21–30 | |||
| Karyotype a | Good | Intermediate | Poor | — | — | |||
| Cytopenias b | 0/1 | 2/3 | — | — | — | |||
| Definition of the individual patient’s risk group | ||||||||
| Risk Group | Score | Median Survival (Years) | Median Times to 25% AML Evolution | |||||
| Low | 0 | 5.7 | 9.4 | |||||
| Intermediate 1 | 0.5–1.0 | 3.5 | 3.3 | |||||
| Intermediate 2 | 1.5–2.0 | 1.2 | 1.1 | |||||
| High | ≥2.5 | 0.4 | 0.2 | |||||
a Good: normal, -Y, del(5q), del(20q); poor: complex (≥3 abnormalities) or chromosome 7 anomalies; intermediate: other abnormalities.
b Cytopenias were defined as a hemoglobin level of less than 10 g/dL, an absolute neutrophil count of less than 1.5 × 10 9 /L, and a platelet count of less than 100 × 10 9 /L.
In 2001, the WHO formulated a new classification of MDS based on uni- or multilineage dysplasia, bone marrow blast count, and distinctive cytogenetic features. This proposal was shown to provide a relevant prognostic information, and was largely confirmed in the updated classification 2008. In particular, among patients with MDS without excess blasts, an isolated involvement of the erythroid lineage was found to be associated with a better prognosis compared with multilineage dysplasia.
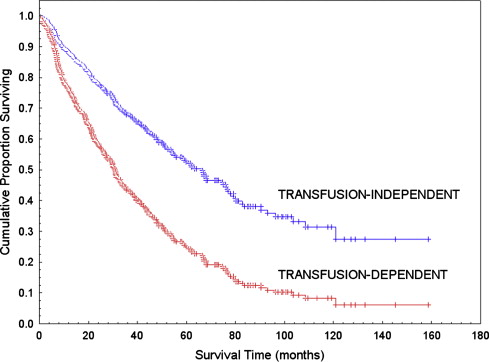
In a study on the prognostic significance of the WHO classification, the authors found that the IPSS retained significance within the WHO subgroups. However, when accounting for blast percentage using WHO categories, the only other IPSS variable adding prognostic information was found to be cytogenetics. The authors also found that transfusion dependency had a significant effect on survival in multivariable analysis. Fig. 1 illustrates the important impact of transfusion dependency on survival of MDS patients. The authors concluded that transfusion dependency could be considered as an independent indicator of the severity of the disease, and consequently an independent prognostic factor. Analyzing pre-transfusion hemoglobin levels in independent cohorts of subjects, transfusion-dependency was shown to recognize a homogeneous population including about 95% of subjects with less than 9 g/dL. These data are in agreement with the results of a recent study by Greenberg and coworkers, indicating that the severity of anemia at diagnosis is of additive prognostic value to IPSS in survival.
Several mechanisms may concur to determine the negative impact of severe anemia on the clinical outcome of patients with MDS. The severity of anemia likely reflects more severe marrow failure and more aggressive disease. In addition, chronic anemia is known to be associated with increased mortality and morbidity per se in older adults, especially when associated with chronic heart failure. Furthermore, cardiac dysfunction can result from transfusional iron overload developing in adulthood.
The World Health Organization classification-based Prognostic Scoring System
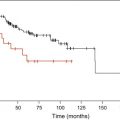
In collaboration with the Duesseldorf colleagues, the authors developed a prognostic model that accounted for the WHO categories, cytogenetics, and transfusion dependency. This WHO classification-based Prognostic Scoring System (WPSS) was able to classify patients into five risk groups showing different survivals and probabilities of leukemic evolution. WPSS was shown to predict survival and leukemia progression at any time during follow-up, and may therefore be used for implementing risk-adapted treatment strategies. Fig. 2 illustrates the risk for progressing from a WPSS risk group to the next one or to acute myeloid leukemia over time.
More recently, the authors found that bone marrow fibrosis identifies a distinct subgroup of MDS with multilineage dysplasia, high transfusion requirement, and poor prognosis, and represents an independent prognostic factor that may be useful in clinical decision making. Within patients stratified according to IPSS and WPSS categories, bone marrow fibrosis involved a shift to a one-step more advanced risk group. As indicated in Table 2 , grade 2 to 3 marrow fibrosis can therefore be used to better define the individual patient’s risk group within the WPSS.
| Calculation of the WPSS Score and Evaluation of Bone Marrow Fibrosis | ||||||
|---|---|---|---|---|---|---|
| Variable | Variable Scores | |||||
| 0 | 1 | 2 | 3 | |||
| WHO category | RA, RARS, MDS with isolated deletion (5q) | RCMD | RAEB-1 | RAEB-2 | ||
| Karyotype a | Good | Intermediate | Poor | — | ||
| Transfusion requirement b | Absent | Present | — | — | ||
| Bone marrow fibrosis c | The presence of grade 2–3 bone marrow fibrosis involves a shift to a one-step more advanced risk group after accounting for WHO category, karyotype, and transfusion requirement. | |||||
| Definition of the individual patient’s risk group | ||||||
| Risk Group | Score | Median Survival (Years) | Risk of Leukemic Evolution | |||
| Very low | 0 | >10 | <10% at 15 y | |||
| Low | 1 (or score 0 plus marrow fibrosis) | >5 | 10%–20% at 5 y | |||
| Intermediate | 2 (or score 1 plus marrow fibrosis) | ∼4 | 30%–40% at 5 y | |||
| High | 3–4 (or score 2 plus marrow fibrosis) | ∼2 | 30% at 3 y | |||
| Very high | 5–6 (or score 3–4 plus marrow fibrosis) | ∼1 | >50% at 1 y | |||
a Good: normal, -Y, del(5q), del(20q); Poor: complex, chromosome 7 anomalies; Intermediate: other chromosomal abnormalities.
b Transfusion dependency: at least one transfusion every 8 weeks over a period of 4 months.
c Bone marrow fibrosis should be evaluated according to the European consensus criteria. Data from Cazzola M, Malcovati L. Risk-adapted treatment of myelodysplastic syndromes. Hematology Education 2009;3:181.
Accounting for multilineage dysplasia, transfusion dependency and bone marrow fibrosis within the WPSS categorization allows defining the prognosis of the individual patient with MDS more accurately as compared with the IPSS categorization. This accuracy is mainly noticeable for patients belonging to the IPSS low or intermediate-1 risk groups ( Table 3 ) (ie, those patients who pose most difficulties in therapeutic decision making).
| IPSS Risk Group | No. of Subjects | Median Overall Survival a | WPSS Risk Group | No. of Subjects (%) | Median Overall Survival b |
|---|---|---|---|---|---|
| Low | 71 | 68 | Very low | 26 (37) | 141 |
| Low | 23 (32) | 66 | |||
| Intermediate | 16 (23) | 48 | |||
| High | 6 (8) | 26 | |||
| Intermediate 1 | 122 | 42 | Very low | 6 (5) | 141 |
| Low | 23 (19) | 66 | |||
| Intermediate | 42 (34) | 48 | |||
| High | 33 (27) | 26 | |||
| Very high | 18 (15) | 9 | |||
| Intermediate 2 | 47 | 14 | Intermediate | 1 (2) | 48 |
| High | 25 (53) | 26 | |||
| Very high | 21 (45) | 9 | |||
| High | 18 | 5 | High | 1 (6) | 26 |
| Very high | 17 (94) | 9 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree