IgM monoclonal gammopathy of any concentration
Bone marrow infiltration by small lymphocytes showing plasmacytic differentiation
Intertrabecular pattern of bone marrow infiltration
Surface IgM+, CD19+, CD20+, CD22+, CD25+. CD27+, FMC7+, CD5±, CD10−, CD23−, CD103−
4 Biology of WM
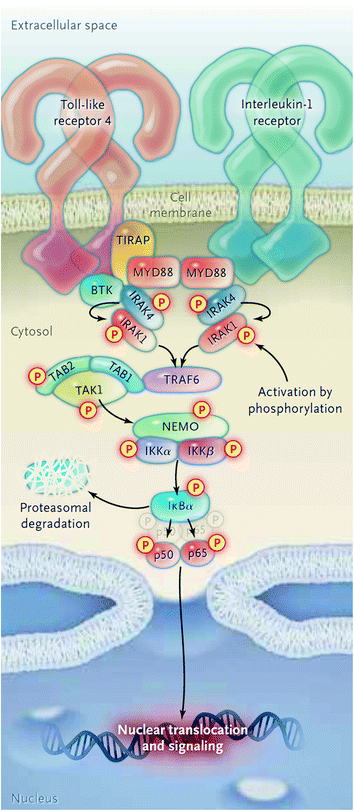
The MYD88 L265P gene mutation has shown to support growth and survival of WM cells in several studies. A knockdown model of MYD88 showed decreased survival of MYD88 L265P expressing WM cells, whereas survival was more enhanced by knock-in of mutant versus wild-type MYD88 [10]. MYD88 acts as an adaptor molecule in toll-like receptor (TLR) and interleukin-1 receptor (IL-1R) signaling [11]. Following stimulation of TLR or IL-1R, MYD88 is recruited to the activated receptor complex as a homodimer which complexes with IL-1R-associated kinase 4 (IRAK4) and subsequently activates IRAK1 and IRAK2 [12]. IRAK1 activation then leads to NF-κB activation via IκBα phosphorylation [13]. Recently, a study has shown that MYD88 L265P also activates the Bruton’s Tyrosine Kinase (BTK) pathway [10]. In this preclinical study, the activation of BTK by MYD88 could be abrogated by the use of BTK kinase inhibitors. A diagram of the activation of BTK and NF-κB via MYD88 is shown in Fig. 1.
A recent study from our group first ever reported the occurrence of recurrent somatic CXCR4 gene mutations in approximately 30 % of WM patients [14]. The somatic mutations occur in the C-terminal domain and are similar to those observed in patients with WHIM (Warts, Hypogammaglobulinemia, Infections, and Myelokathexis) syndrome. These mutations regulate signaling of CXCR4 by its ligand SDF-1a [15]. In WM patients, two classes of CXCR4 mutations occur: non-sense (CXCR4WHIM/NS) and frameshift (CXCR4WHIM/FS) mutations [14, 16]. Non-sense and frameshift mutations are almost equally divided among WM patients, and over 30 different types of CXCR4 mutations have been identified. Preclinical studies with the most common CXCR4 S338X mutation in WM have shown sustained signaling of AKT, ERK, and BTK following SDF-1a binding in comparison with wild-type CXCR4, as well increased cell growth and survival of WM cells [17]. Figure 2 shows (a) the protein sequence for the full-length transcript, (b) the crystal structure of CXCR4, and (c) the location of the WHIM-like mutations.
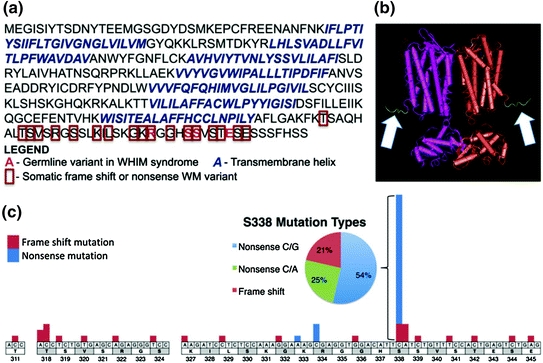

Fig. 2
CXCR4 full-length transcript and structure, and the location of WHIM-like mutations. Reproduced with permission from [14]
5 Criteria for Initiation of Therapy
Given the indolent and incurable nature of WM, a large proportion of patients would not need immediate therapy upon diagnosis and will be placed on watchful waiting. Current guidelines do not recommend initiation of therapy based on IgM levels alone since they might not correlate with clinical manifestations of the disease [18]. Initiation of therapy is, however, reasonable in patients demonstrating rising IgM levels along with signs or symptoms associated with disease progression. Criteria for initiation of therapy are shown in Table 2. In patients in whom an immediate control of the disease is warranted, such as symptomatic hyperviscosity, coagulopathy, cryoglobulinemia, or cold agglutinin disease, a rapid reduction of the IgM paraprotein should be achieved with plasmapheresis. Treatment directed at WM should follow as soon as possible since plasmapheresis does not constitute definitive therapy and IgM levels will rise and return to baseline levels within 4 weeks.
Table 2
Criteria for initiation of therapy in Waldenström macroglobulinemia
Constitutional symptoms (i.e., fever, night sweats, fatigue due to anemia or weight loss) |
Progressive, symptomatic lymphadenopathy or splenomegaly |
Anemia with a hemoglobin ≤10 g/dl |
Platelet count <100 × 109/L |
Hyperviscosity syndrome |
Symptomatic sensorimotor peripheral neuropathy |
Systemic amyloidosis |
Symptomatic cryoglobulinemia |
6 Frontline Therapy for WM
There are several options for the frontline therapy of patients with WM. These options include alkylating agents (i.e., chlorambucil, cyclophosphamide, and bendamustine), nucleoside analogs (i.e., fludarabine and cladribine), the immunomodulator thalidomide, and the proteasome inhibitor bortezomib, with or without the anti-CD20 monoclonal antibody rituximab [19]. Individual patient considerations should be taken into account when making the choice of frontline treatment including the presence of cytopenias, need for rapid disease control, age, pre-therapy IgM levels, pre-existing neuropathy, and candidacy for autologous transplantation therapy. For candidates for autologous transplantation, exposure to alkylator therapy or nucleoside analogs should be limited because of the difficulties with stem cell collection. The use of nucleoside analogs should be approached cautiously given that increased risk of disease transformation, myelodysplasia, and acute myeloid leukemia have been reported.
6.1 Proteasome Inhibitor-Based Therapy
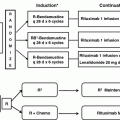
The WM Clinical Trial Group presented data on a study that included 23 patients using bortezomib, dexamethasone, and rituximab (BDR) for the primary therapy of symptomatic WM [20]. Bortezomib was administered at a dose of 1.3 mg/m2 IV along with dexamethasone 40 mg on days 1, 4, 8, and 11 and rituximab 375 mg/m2 IV on day 11 every 21 days for four induction cycles, followed by four maintenance cycles administered every 3 months. The overall response rate (ORR) was 96 % with three complete responses (CR), two near complete responses (nCR), and three very good partial responses (VGPR) for a VGPR or better of 35 %. The median time to response was 1.4 months, which makes BDR an excellent choice if a rapid control of disease is required or in young patients to decrease the risk of secondary myeloid malignancies. With a median follow-up of 23 months, 18 out of 23 patients (78 %) remained free of progression. The most common toxicity was peripheral neuropathy; 39 and 30 % of patients, respectively, experienced grade 2 and grade 3 peripheral neuropathy. Grade 2 and grade 3 neutropenia were also reported in 26 % and 26 % of patients, respectively. It is important to note that appropriate Herpes zoster prophylaxis should be instituted in all patients receiving proteasome inhibitor therapy.
Another study evaluated weekly bortezomib in combination with rituximab in 26 untreated patients with WM [21]. In this study, bortezomib was administered at a dose of 1.6 mg/m2 IV weekly on days 1, 8, and 15 every 28 days for six cycles with rituximab 375 mg/m2 weekly times four during cycles 1 and 4 only. The ORR was 88 % with a VGPR or better of 8 %. The median time to best response was 3.7 months. The 1-year PFS rate was 75 %. There was 54 % of peripheral neuropathy grade 2 or lower but no grade 3 or higher neuropathy was observed. Grade 3 or higher neutropenia was seen in 12 % of patients. More recently, a study by the European Myeloma Network evaluated the efficacy of weekly bortezomib, low-dose dexamethasone, and rituximab in 59 untreated WM patients [22]. Bortezomib was administered as a single agent at 1.3 mg/m2 IV on days 1, 4, 8, and 11 (cycle 1) followed 3 weeks later by weekly bortezomib at 1.6 mg/m2 IV and dexamethasone 40 mg IV on days 1, 8, 15, and 22 and rituximab 375 mg/m2 IV every 35 days (cycles 2–5). The ORR was 85 % with VGPR or better of 10 %. After a 32-month follow-up, the median PFS was 42 months. Grade 3 or higher peripheral neuropathy occurred in 7 % of patients. The subcutaneous (SQ) administration of bortezomib has shown to be effective and induces less neuropathy than the IV route in patients with myeloma [23]; however, the safety and efficacy of bortezomib SQ has not been formally studied in patients with WM.
A recent study evaluated the combination of carfilzomib, dexamethasone, and rituximab (CaRD) in 31 patients with WM [24]. Carfilzomib was administered at a dose of 20 mg/m2 IV during cycle 1 and at 36 mg/m2 IV during cycles 2–6 with dexamethasone 20 mg IV on days 1, 2, 8, and 9 along with rituximab on days 2 and 9 every three weeks during induction therapy. Maintenance therapy consisted on carfilzomib 36 mg/m2 IV and dexamethasone 20 mg IV on days 1 and 2 and rituximab 375 mg/m2 on day 2 every 2 months for eight cycles. The ORR was 87 % with one CR and ten VGPR for a VGPR or better of 35 %. The 15-month PFS rate was 65 %. No grade 3 neuropathy was observed. Grade 3 hyperglycemia and hyperlipasemia were observed in 23 and 16 % of patients, respectively. Of note, the rate of IgG hypogammaglobulinemia increased from 48 % at baseline to 90 % following therapy.
6.2 Alkylator-based Therapy
Cyclophosphamide-based therapy includes regimens in which cyclophosphamide is combined with rituximab and prednisone (CP-R), rituximab, prednisone and vincristine (CVP-R), and rituximab, prednisone, vincristine and doxorubicin (CHOP-R). These regimens would induce an ORR of 70–80 % with CR rate around 10 % [25]. It is unclear if the addition of vincristine or doxorubicin translates into clinical benefits in patients with WM. A retrospective study compared the activity and toxicity of CP-R, CVP-R, and CHOP-R in patients with WM [26]. In that study, CP-R was associated with similar response rates than CVP-R and CHOP-R. However, the rate of treatment complications such as febrile neutropenia, hospitalizations, and neuropathy was lower. Based on these data, CP-R might be preferred over CVP-R or CHOP-R in patients with WM.
The combination of bendamustine and rituximab (BR) has been compared with CHOP-R in a randomized controlled study by the Study Group for Lymphomas in a cohort that included 42 patients with WM [27]. Bendamustine was administered at a dose of 90 mg/m2 IV on days 1 and 2 along with rituximab 375 mg/m2 IV on day 1 at 3-week intervals for 6 cycles. The ORR for BR was 96 % and 94 % for CHOP-R. With a median follow-up of 26 months, the PFS rate for BR was 90 % compared with 59 % for CHOP-R. BR was associated with lower rates of grade 3 or higher neutropenia, infectious complications, and alopecia, but with higher rates of skin rash. BR might provide an excellent therapeutic option for patients with lymphadenopathy or patients with pre-existing neuropathy.
6.3 Nucleoside Analog-Based Therapy
In the large randomized controlled study to date, which enrolled 414 patients (339 WM, 37 MZL, and 38 LPL) from 101 centers in five countries, fludarabine was compared with chlorambucil in untreated WM patients [28]. In this study, the ORR for fludarabine was 48 % versus 39 % for chlorambucil. The median PFS was also longer for fludarabine (36 months) in comparison with chlorambucil (27 months). There was a significant improvement in median OS, which was not reached for fludarabine versus 70 months for chlorambucil. Finally, the rate of second malignancies was higher in the chlorambucil arm than in the fludarabine arm (21 % vs. 4 %).
The long-term outcomes of the combination of fludarabine and rituximab (FR) were evaluated in 43 patients with WM, from which 27 patients were untreated [29]. Therapy consisted of 8 infusions of rituximab at 375 mg/m2 IV per week administered at weeks 1–4, 17, 18, 30, and 31, along with 6 cycles of fludarabine 25 mg/m2 IV daily given for 5 days at weeks 5, 9, 13, 19, 23, and 27. The ORR in untreated patients was 96 %. The median time to response was 3.9 months, and the median time to best response was 19 months. The median time to progression in untreated patients was 78 months. Grade 3 or higher adverse events included myelosuppression and infections. Other observed complications were aggressive transformation and secondary malignancies, although most of these events were seen in the previously treated group of patients.
It is unclear at this time whether the combination of an alkylating agent such as cyclophosphamide, a nucleoside analog such as fludarabine, and rituximab (FCR) provides any additional benefits in patients with WM. A study evaluated FCR in 43 patients with WM of which 28 were previously untreated [30]. The FCR regimen consisted of rituximab 375 mg/m2 IV on day 1 and fludarabine 25 mg/m2 and cyclophosphamide 250 mg/m2 IV on days 2 through 4. Cycles were repeated every four weeks for a maximum of six cycles. The ORR was 79 % with no difference between untreated and previously treated patients. Grade 3 or higher neutropenia was seen in 88 % of the patients. After a median follow-up of 39 months, 11 patients (25 %) have died, including five from progressive disease, three from pneumonia, and one from acute myeloid leukemia.
6.4 Other Options
The combination of thalidomide and rituximab (TR) has been associated with an ORR of 70 % and a median PFS of 3 years [31]. TR could be useful in myelosuppressed patients in whom an immediate disease control is not required. The main adverse event associated with thalidomide is neuropathy, which can be seen in up to 40 % of patients treated with TR.
The use of rituximab as single agent can be considered in selected patients with low IgM levels or concurrent autoimmune processes such as cold agglutinin disease, autoimmune thrombocytopenia, or IgM-related neuropathy. The ORR to 4 weekly infusion of rituximab is 20–30 % [32] and to 4 weekly infusions followed by 4 weekly infusions 12 weeks later of 40–50 % [33]. The use of single-agent rituximab in patients with high IgM levels has been associated with IgM flares with an occurrence rate of 40–50 % [34]. These flares might lead to symptomatic hyperviscosity as well as worsening IgM-related neuropathy or cryoglobulinemia. Hence, rituximab monotherapy should be avoided in patients with IgM levels >4,000 mg/dl.
7 Salvage Therapy for Relapsed/Refractory WM
For those patients with relapsed/refractory disease, any of the frontline therapies mentioned above are appropriate options. Therapies in the relapsed setting should also be personalized to the individual patient considering age, performance status, pre-existing neuropathy, IgM levels, and/or need for immediate control of the disease. One must also have in mind to avoid the use of stem cell toxic therapy in patients in whom high-dose chemotherapy with autologous stem cell rescue is being considered.
7.1 Bortezomib
Bortezomib-based therapy has shown to be effective in relapsed or refractory WM patients in several studies, either alone or in combination with rituximab [35–38]. The ORR ranged between 30 and 80 % depending on the study with the most common adverse events being peripheral neuropathy, neutropenia, and thrombocytopenia. Once-weekly administration of bortezomib has been associated with lower rates of neuropathy when compared with the twice-weekly regimen. In one study, bortezomib seemed to be less effective on inducing lymph node responses [35]. As mentioned previously, patients undergoing therapy with proteasome inhibitors should receive prophylaxis against herpes zoster.
7.2 Nucleoside Analogs and Alkylating Agents
Fludarabine has single-agent activity in relapsed WM with an approximate 40 % ORR [39]. The combination FR showed high activity in relapsed/refractory WM patients with ORR of 93 % [29]. The most common adverse events were neutropenia and infections. There were also concerns about aggressive transformation and the development of secondary myeloid malignancies.
The combination BR showed an ORR of 83 % with a median PFS of 13 months [40]. The most common adverse events were prolonged myelosuppression, specifically in patients who had received prior nucleoside analog therapy.
7.3 Ofatumumab
The fully human anti-CD20 monoclonal antibody ofatumumab is also useful in the relapsed/refractory setting. Ofatumumab has shown to be effective and well tolerated in WM patients who develop rituximab intolerance [40]. A study published in abstract form showed an ORR of 57 % in patients with relapsed/refractory WM [41]. The occurrence rate of IgM flare to ofatumumab in this study was 5 %. The most common adverse events were infections.
7.4 Everolimus
Everolimus is an oral mammalian target of rapamycin inhibitor and has induced an ORR of 50 % in patients with relapsed/refractory WM [42]. The median time to response in patients who achieved a PR was 2 months with a median PFS of 21 months. The most common adverse events were anemia, leukopenia, thrombocytopenia, and stomatitis. Everolimus has also been studied in the frontline setting [43]. In this multicenter prospective study, 33 WM patients who were not previously treated received everolimus at a dose of 10 mg PO once daily. The best ORR was 72 % with a median time to best response of 6 months. Interestingly, the bone marrow burden of disease remained unchanged. This bone marrow–IgM discordance complicated response assessment. The most common adverse events were anemia, oral ulcerations, and pneumonitis.
7.5 Ibrutinib
The oral Bruton tyrosine kinase inhibitor ibrutinib has been evaluated in 63 patients with relapsed/refractory WM [44]. Ibrutinib was administered at a dose of 420 mg PO daily. The ORR was 81 % with a median time to response of 4 weeks. The most common adverse events were thrombocytopenia and neutropenia. Of note, WM patients with CXCR4 gene mutations experienced lower response rates to ibrutinib than patients with wild-type CXCR4.
8 Maintenance Therapy
A retrospective study examined the outcome of 248 WM rituximab-naïve patients who were treated with rituximab-containing regimen and then either observed or received maintenance rituximab [45]. In this study, further improved responses after induction therapy were seen in 10 % (16 out of 162) of observed patients and in 42 % (36 out of 86) of patients who received maintenance rituximab. Both PFS (56 vs. 29 months) and OS (>120 vs. 116 months) were longer in patients who received maintenance rituximab. Improved PFS was evident despite previous treatment status or type of induction therapy. Among patients receiving maintenance rituximab, an increased number of infectious events, predominantly grades 1 and 2 sinusitis and bronchitis, were observed. A prospective study examining the role of maintenance rituximab in patients with WM has been initiated by the German STiL group [46]. After undergoing induction therapy with BR, 100 out 162 patients responded to BR (ORR 86 %). From these patients, 43 were then randomized to observation and 47 to maintenance rituximab. Enrollment for this study is complete, and final results are awaited.
9 High-Dose Therapy and Stem Cell Transplantation
The use of stem cell transplantation (SCT) therapy has been explored in patients with WM. Several small series have been reported for autologous and allogeneic transplantation, with variable outcomes. Kyriakou and colleagues reported the largest experience using European Bone Marrow Transplant (EBMT) data for WM patients receiving autologous SCT [47]. Among 158 relapsed/refractory WM patients receiving an autologous SCT, the 5-year PFS and OS rates were 49 and 69 %, respectively. Non-relapse mortality at 1 year was 4 %. Chemorefractory disease and number of prior lines of therapy at time of autologous SCT were the most important prognostic factors for PFS and OS. When used as consolidation at first response, autologous SCT provided a PFS rate of 44 % at 5 years. Long-term outcomes of WM patients undergoing allogeneic SCT from EBMT have been reported [48]. A total of 86 patients received allograft by either myeloablative or reduced-intensity conditioning (RIC). The median age was 49 years, and 47 patients had 3 or more previous lines of therapy. Eight patients failed prior autologous SCT. Fifty-nine patients (69 %) had chemotherapy-sensitive disease at the time of allogeneic SCT. Non-relapse mortality rate at 3 years was 33 % for patients receiving myeloablative transplant and 23 % for those who received RIC. The ORR was 76 %. The relapse rates at 3 years were 11 % for myeloablative and 25 % for RIC recipients. Five-year PFS and OS rates for WM patients who received a myeloablative allogeneic SCT were 56 and 62 %, respectively, and for patients who received RIC were 49 and 64 %, respectively. The occurrence of chronic graft-versus-host disease was associated with improved PFS and suggested the existence of relevant graft-versus-WM effect in this study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree