17 Probiotic Therapy
17 Probiotic Therapy
 Introduction
Introduction
The objective of probiotic therapy is to optimize the body’s defense mechanisms with microbiotics and autovaccines and, possibly, to replace antibiotics with probiotics.
Probiotics are increasingly becoming established as a therapeutic option in addition to antibiotics. Antibiotics and probiotics are not necessarily antagonistic. Antibiotics are still the last resort in cases of severe bacterial or parasitic infections. Positive effects of antibiotics can be maximized and negative effects on the immune system minimized by strictly controlling their application.
Probiotics are useful therapeutic tools in numerous cases of acute and, above all, chronic infections. Prerequisite for applying probiotics is the critical delimitation of life-threatening infections or imminent complications. Probiotics can reduce the negative effects of antibiotics, e. g., problems posed by resistance, and optimize their positive effects on immunological functions.
The International Study Group on New Antimicrobial Strategies (ISGNAS) defines three categories of probiotics (5, 16, 17, 21):
• Medical probiotics: microbial preparations (microbiotics) that contain live and/or inactivated (dead) microorganisms—including their constituents and products—and are used for therapeutic purposes. These bacterial preparations and autovaccines are used as remedies, the efficacy of which has been documented.
• Pharmaceutical probiotics: microbial preparations that are used as food additives. As a rule, these are products manufactured under pharmaceutical conditions; they contain lyophilized microorganisms in high concentrations and are available at pharmacies.
• Alimentary probiotics: microbial preparations that play a role in food fermentation or food production. Alimentary probiotics are usually dairy products, such as yogurt. Probiotic dairy products can play a useful role in nutrition. However, despite assertion to the contrary, yogurt cannot be considered a medicine.
We focus here on the use of symbiotic microorganisms in the form of medical and pharmaceutical probiotics that can control complex immune mechanisms, modulate the endogenous microflora interactively, and control metabolic processes.
Man and Microbes
Homo sapiens represents only a snapshot in evolution and will continue to evolve in the future. From the very beginning, microbes played an important role. Mutual dependencies and requirements developed, and these are reflected in complex functional areas and diverse differentiations of structural interrelationships. The term symbiosis is often used in this connection. However, the definition of symbiosis includes a range of partnerships, such as parasitism, neutralism, commensalism, and mutualism. In a partnership such as parasitism, only one of the partners is the beneficiary, namely the parasite, whereas the other partner is harmed by the parasite. A symbiotic association in which partners coexist without mutual interaction and without obvious advantages or disadvantages is called neutralism. Commensalism is a close association from which only one of the two partners benefits without causing harm to the other partner. A partnership in which both partners benefit from each other is called mutualism (mutualistic symbiosis – coexistence for mutual benefit).
Gnotobiology
Much of our knowledge on the interaction between unicellular and multicellular organisms comes from gnotobiology, the science dedicated to studying animals raised in a germ-free environment, comparing them with microflora-associated normal animals, and also studying monoflora-associated or polyflora-associated animals.
We distinguish between microflora-associated characteristics (MAC) and germ-free animal characteristics (GAC). There are essential differences between conventional, microflora-associated, and germ-free animals. Germ-free animals are not able to defend themselves effectively against infections. Their association with pathogenic microorganisms is lethal. This can be attributed to the fact that, in germ-free animals, essential components of the complex immune system are underdeveloped and function only to a limited extent or not at all. In particular, the mucosa-associated immune system is developed only to a rudimentary degree. The intestine-associated lymph tissue is particularly affected. The lymph vessels in the intestinal wall (lacteal vessels) are hardly present, or not at all. Peyer’s plaques and lymph follicles are smaller and intraepithelial T lymphocytes are severely reduced in numbers. The effectivity of immune cells is greatly decreased or does not exist at all. Serum immunoglobulin levels are extremely low.
Table 17.1 Positive effects of the intestinal microflora
Establishment of a microbial barrier
• Resistance to colonization: the dominance of autochthonous microbes in intestinal biotopes and microecologic niches inhibits colonization by heterogenous germs or by invasive or toxic microorganisms (e.g., inhibition of Candida, Clostridium difficile, Staphylococcus aureus, and others)
• Competition for nutrients, vitamins, and other essential factors
• Acidification of the intestinal milieu (lactic acid, acetic acid)
• Production of microbicidal or microstatic substances that inhibit the growth of pathogenic germs (bacteriocins, short-chain fatty acids, hydrogen sulfide, hydrogen peroxide)
• Adhesion of friendly microbes to mucosal receptors prevents colonization by pathogenic invaders
Training of the immune system
• Development and differentiation of lymphatic organs
• Function of the immune system, especially of the mucosal immune system
• Immunoregulation (immunomodulation, immunostimulation, immunosuppression)
Intestinal motility
• Stimulation of peristalsis
• Blood supply to the intestinal mucosa
Effect on epithelia
• Proliferation of epithelial cells
• Nutritional supply of epithelial cells by short-chain fatty acids as energy source for the colon’s epithelium
Vitamin production
• Vitamin K
• Vitamin B12
• Folic acid
• Nicotinamide
The structure and function of all components of the defense system of multicellular organisms depend therefore directly on the microbial populations at various locations on skin and mucosa, but primarily in the entire intestinal tract. Many other factors are influenced by the presence of the microflora (Table 17.1).
Microbial Populations
The structures of the mucosa as interface and the colonization of this surface by symbiotic microorganisms represent a phylogenetically and ontogenetically determined functional unit and are part of the innate immune system. In stable layers of microbial populations, such as the intestinal flora, mutualistic and commensal microorganisms are in the majority. Together with the immune system, this majority keeps the minority of opportunists under surveillance and usually restricts the number of germs.
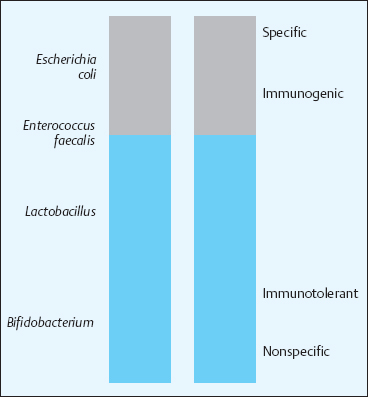
The most common intestinal microorganisms are Bifidobacterium, Eubacterium, Lactobacillus, and other genera that ensure the stability of the microbial populations. Elements of instability include many species of Enterobacteriaceae, Pseudomonas, and Clostridium. An intermediary position is taken by Escherichia coli, Enterococcus, and Bacteroides, which have positive as well as negative properties. The minorities in the microbial populations, however, have by far the broadest effects on the range of defensive functions (Fig. 17.1).
The affected functions concern the entire spectrum from innate to adaptive immunity, from nonspecific to specific immune reactions, and they control both mucosal and systemic immunity. Bifidobacterium and Lactobacillus are accepted by the host as “friendly” inhabitants and stimulate the intestine-associated lymphatic system with their predominantly nonspecific activities and functions (Fig. 17.2).
Microbial populations play a decisive role in health and disease. The continuous interactions between individual members of the complex microbial populations stabilize this “social community” in a dynamic equilibrium, and it is the stability or instability of this equilibrium that benefits or harms the human body. Normally, the coexistence of the human body with its own microflora is harmonious throughout life. Endogenous microorganisms mediate important protective functions:
Fig. 17.1 Profile microflora and immune system
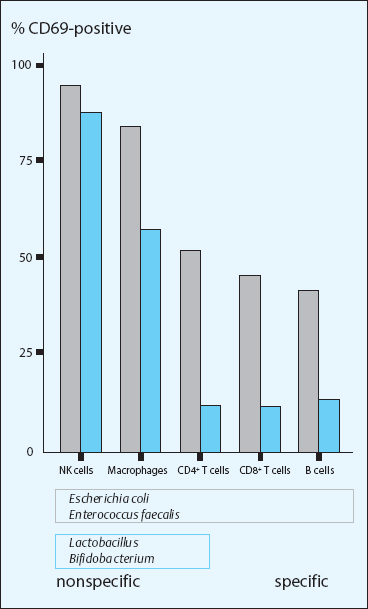
Fig. 17.2 Immunological reaction profiles
– resistance to colonization: the control over colonization with endogenous, facultative pathogenic germs and heterogenous invaders
– stimulation and stabilization of epithelial integrity
– stimulation and differentiation of lymphatic organs.
Any external or internal disturbance of the well-balanced equilibrium of the unicellular microbes with the multicellular body of Homo sapiens has more or less severe consequences for physiological functions and health.
Pathogenic Microbes
A typical sign of the pathogenicity of microorganisms is their ability to break through the barriers of the innate defense system and to propagate in the internal milieu. In the classic case, these are natural pathogens or obligatory pathogens that are normally not members of the partnership between man and microbes, i. e., they are nonsymbionts. The adaptive immune system is responsible for dealing with the assault by such pathogens.
A microorganism can develop pathogenic properties only when it possesses genes that code for pathogenicity factors and when certain conditions lead to the expression of these genes. In the genomes of many pathogenic species of bacteria, transfer and recombination processes have established certain genetic structures that are called pathogenicity islands. These are DNA fragments that occur as genomic islands also in many non-pathogenic microbes. Their genetic codes include information for metabolic functions, secretion processes, or resistances. In terms of evolutionary biology, such genetic islands may be advantageous for microorganisms under certain conditions of selection.
Microorganisms in the environmental reservoir, as well as in symbiotic microbial populations, are constantly involved in the exchange of genes. Pieces of genetic information are continually mixed through horizontal gene transfer, and new microbial variants are thus formed.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree