Disincentives for reporting adverse events
Poor education about what constitutes an event
Concern over legal or credentialing consequences
Personal shame
Fear of implicating others
Time-consuming processes
Systems that are difficult to access
Lack of anonymity
Potentially discoverable information
Slow infrastructure
Arduous, poorly designed interfaces
Lack of feedback and follow-up, no perceived value to the department
Factors that incentivize reporting |
Secure and non-discoverable data |
Quick entry time (less than 1 min) and ease of use |
Accessibility of the system |
The capture of both near misses and incidents of patient harm |
An option of anonymity for entering reports |
Data searchable by the department QI committee |
Summary reports to department and hospital |
A culture of learning, not blame, in response to reports |
Quality Metrics
Quality metrics can be grouped into volume, structure, outcomes, or process measures [7]. All of these have quality and safety implications, and it is important to understand each in the context of an overall safety program.
Volume, in and of itself, may be a driver of safer care. The basic concept is that if an organization has a higher volume, that quality will be higher than a comparable institution with a lower volume of the disease or procedure in question. This ratio has been demonstrated in numerous cases, but is not universal [22].
Structural metrics are defined as the presence or absence of a service, designation, or other designation. An example is the trauma classification of a hospital, for example, Level 1 status. While there may be an association between this and the safety and quality of the trauma program, there is typically not robust evidence that these markers are directly tied to safer outcomes.
Outcomes are probably the most sought-after metric by the public as an overall marker of safe, effective care. However, defining outcomes is exceptionally difficult. There are many confounding variables, such that a simple comparison between facilities may not yield accurate results. Many outcome measures are derived from administrative data sets, which are typically derived from coding and billing data. This is fraught with opportunities for bias. This variability in what in many cases is a manual process then confounds any meaningful attempt at risk adjustment. Patient characteristics are variables that have to be controlled in order to generate meaningful outcome measures. However, no standard approach is available for risk or severity adjustment, and typically these adjustments are based on the aforementioned administrative data sets, further compounding any inherent biases. There are examples where the data is analyzed in such a controlled manner as to draw meaningful conclusions, such as the outcomes for children with congenital heart defects. In 2015, the Society of Thoracic Surgeons (STS) released its assessment of outcomes at hospitals that perform pediatric congenital heart surgery. However, even with robust data collection, analysis, and risk adjust techniques, the results are limited—the centers are represented by a star classification of 1–3 and basic adjusted mortality information only [23].
Process measures assess whether the defined process or standard of care was provided to the patient. These measures are typically abstracted from the medical record rather than from administrative coding data. Provided the compliance with the process is linked to improved outcomes, these measures can add value. For example, a “bundle” exists for central line placement, and following the steps in the process has been linked with a lower CLABSI rate. An example of a process measure is if the clinician was compliant with the bundle. However, it is not an uncommon occurrence for a process measure and an outcome to be discordant. This can be due to confounding variables, or that the process steps are not linked with the outcome.
Trigger tools are an example of a quality metric and an innovative method for measuring the frequency of adverse events. This method consists of performing time-limited screening of charts to find the presence of “triggers” that indicate the possibility of their having been an adverse event. Examples would include the prescribing of reversal agents (e.g., naloxone, diphenhydramine) that may be indicative of an adverse drug event, unplanned return to the operating room that might indicate a surgical complication, etc. In addition to manual chart review approaches to finding and measuring triggers, there are examples now of using automated data mining from electronic medical records to do this. Detailed information on how to use trigger tools to measure harm frequency is beyond the scope of this chapter but available to the interested reader [24, 25].
Adverse Event Management
Management of an adverse event occurs in three phases: mitigation, management, and follow-up, including both disclosure and analysis/improvement efforts.
Mitigation is the management of an ongoing adverse event to prevent or minimize resulting harm to the patient. The specific response depends upon the nature of the situation. For example, if a patient is demonstrating signs of an adverse reaction to a transfusion, the transfusion is discontinued and treatment given for fever or other signs and symptoms. In some cases, the mitigation may be urgent, for example, after an overdose of a medication in which a patient is demonstrating potentially life-threatening reactions (e.g., respiratory depression after opiates). It is often possible to start mitigating an adverse event when warning signs of a potential or an impending adverse event first appear. An adverse event that does not reach the patient because of early mitigation is defined as a near miss.
A good plan can help the provider to anticipate an adverse event and also enables him or her to determine that there has been a deviation from the plan that requires further action. This in turn allows the contingency plan to be activated. As a result, an impending adverse event can be recognized more quickly, and a pre-formulated contingency plan invoked to mitigate further development of the incident. This may not prevent the adverse event from occurring but may help to mitigate the severity of the outcome.
Consider, for example, unanticipated anaphylaxis to intramuscular PEG-asparaginase. During the pre-chemotherapy briefing, the nurse states that the patient had previously received PEG-asparaginase and did not have a reaction to it. Although the patient does not have a known allergy, anaphylaxis medication should be preordered and at the bedside in case an allergic reaction occurs. If the patient develops a small local reaction including respiratory distress, diphenhydramine should be administered. If the patient develops a systemic allergic reaction, diphenhydramine, corticosteroids, and epinephrine should be administered rapidly. In addition, a rapid response alert should be sent to the appropriate intensive care staff. Finally, if the patient develops respiratory failure despite treatment, the airway management protocol should be initiated. The precise triggers should be set for an individual patient, while the concept is that of escalating the care and management.
In summary, mitigation usually involves three steps: attempting to prevent the event from getting any worse, attempting to improve the situation, and making a diagnosis so that further management can be targeted to a specific cause.
Management follows mitigation and starts with diagnostic assessments and/or therapies directed at managing the presumed diagnosis or complication. The word presumed is used because the mode of presentation of the event might lead to an incorrect working diagnosis. This is not a reflection upon the skill or decision-making of the responder, merely, that it is often not possible to make the correct diagnosis until the event has evolved. During the initial management, therefore, one must accept that initial pathways might not be ideal. Until a definitive diagnosis is made (and possibly even afterward), the responder should be aware of alternative possibilities and be prepared to change the working diagnosis.
Imagine for a moment an adolescent patient with lymphoma who has been admitted for fever and neutropenia and is stable on antibiotics. Twenty-four hours into his hospitalization, the nursing staff identify that the patient is wheezing. Albuterol is prescribed for a presumed exacerbation of underlying asthma. If, shortly thereafter, the patient develops urticaria and hypotension, the diagnosis of anaphylaxis would be readily considered. It may initially present with bronchospasm and then be treated according to a specific management protocol, but other causes of bronchospasm should be considered and excluded while this treatment is commenced. In a different situation, the correct diagnosis might be either bronchospasm or anaphylaxis, but this example highlights the importance of keeping an open mind during the management of an adverse event. On occasion a diagnosis is not apparent, or initial efforts at management are insufficient. If a “call for extra help” has not already been made, this is the stage where consultation is appropriate. An example is the initiation of an RRT (rapid response team). Each hospital, clinic, and infusion area should have a mechanism for summoning additional resources, and in many hospitals, patients and family members are informed about their ability to request an emergency response team if they feel their concerns are not being addressed.
Follow-up after adverse occurrences consists of the management of the residual clinical conditions, disclosure, learning from outcomes, improvements in patient safety, as well as risk management and medicolegal considerations. Another follow-up to consider is reporting to an event registry because this may trigger analysis and studies that lead to prevention of the initial event. The reporting may be through an incident report, M&M process, or other means—but a sign of a strong safety culture is that cases of harm or near misses are always reported and evaluated for improvement.
Disclosure After Adverse Events
After an adverse event occurs, consideration should be given to disclosing the event to the patient and family. Multiple studies have shown that physicians and patients believe that when a medical error occurs, it should be disclosed in a timely fashion [26, 27]. Further, in many circumstances, there are benefits to the provider by disclosing the adverse event. According to Robbennolt, a physician, attorney, and expert in medical error, “Patients were less likely to indicate they would seek legal advice when the physician assumed responsibility for the error, apologized, and outlined steps that would be taken to prevent recurrence” [28]. In 2001 the University of Michigan formed an institutionally supported program of full disclosure, investigations, a timely apology, implementation of solutions to prevent the error, and, when appropriate, financial compensation in response to medical errors. This program showed a statistically significant decrease in claims, compensation, and legal costs [29]. Even given agreement on the responsibility to disclose, and potential benefits, multiple studies illustrate that disclosure is not assured [30–32]. Providers are less likely to disclose when the error did not have a tangible effect on the patient’s outcome [26, 33]. Barriers to disclosure are legal and financial factors, the physician’s reputation, an expectation of perfection by patients and physicians, emotional distress, and a medical culture that focuses on personal responsibility rather than the effect of system-based errors [33]. Implementing an educational program at the department and/or hospital level can reduce these barriers.
A recommended approach to disclosure is summarized below:
- 1.
Contact the hospital’s risk management department for support if the practitioner is uncertain of the best practices surrounding event disclosure.
- 2.
State the facts of what happened and that a medical error occurred.
- 3.
Offer an apology.
- 4.
Review the clinical implications of the medical error.
- 5.
Assure the patient the reasons for the error will be investigated and that the patient and family will be informed of the results and how the institution will prevent the error from recurring.
- 6.
Consider waiving charges or offering compensation when appropriate.
Event Review
The goal of event review is to identify the proximate and/or root causes of errors that contribute to adverse events. These errors may be individual human mistakes, but often they are due to underlying systems issues (latent errors) that could lead to future harm. Identifying and addressing the latent errors in the system is a far more effective strategy than simply trying to retrain or write new policies to prevent future human errors.
Root Cause Analysis
A variety of tools have been used to analyze and manage hazards and incidents in healthcare, including various forms of root cause analysis (RCA) or apparent cause analysis (ACA). The Joint Commission requires an RCA to be performed for all adverse events that reach the patient and cause severe temporary harm requiring intervention, permanent harm, or death (sentinel events) [34]. Root cause analysis typically includes individual interviews of all team members involved in any serious safety event by two neutral facilitators, trained in interviewing and sensitive to principles of just culture. Facts and perspectives gathered through these discussions are then summarized by the interviewers and presented to a group of peers and/or supervisors of the staff involved in the event. The purpose of this “root cause analysis team” is to identify the proximate and root causes of the occurrence and recommend countermeasures that, if implemented, can prevent a future recurrence. These RCA teams are generally supported by an executive level sponsor who is accountable for the results of the review and the implementation of the action plans. Results of RCAs should be reviewed with appropriate governance committees overseeing organizational safety, and they should ensure timely and effective implementation of action plans.
The relationship between hazards, barriers, and incidents, which may be identified in an event review, is typically complex and difficult to represent in a concise manner. Moreover, root cause analysis can be very time-consuming and is not an appropriate method for analyzing the majority of events and near misses that are reported to a voluntary registry. A number of cognitive aids have been developed to show the causes of an incident including the use of causal trees [34]. Ideally the results of an event review should be depicted in a diagram, so that the accident trajectory can be understood. Diagrams for depicting the accident trajectory include the Swiss cheese model [35], causal trees [34], and more recently bowtie diagrams [36].
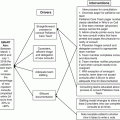
Just Culture Algorithm
The question that drives safety work in a just culture is not who is responsible for failure, rather, it asks what is responsible for things going wrong. What is the set of engineered and organized circumstances that is responsible for putting people in a position where they end up doing things that go wrong? –Sidney Dekker
Another critical part of event review is a determination if the caregivers involved adhered to the standards of care and if not, why? Humans make mistakes, frequently, and human error is unavoidable. A “just culture” is one in which all participants are evaluated in a fair, equitable, and balanced fashion. Tragic examples of dedicated healthcare team members who have been involved in errors that caused serious harm or death of patients who are then “second victims” and are fired, incarcerated, or experience psychological trauma and even harm themselves are the reasons why it is imperative that health system leaders approach adverse occurrences in a just and fair way. There are many similar algorithms for evaluating occurrences using these principles [37]. One such example in use at the authors’ hospital and medical school is included here (Fig. 8.1).