Principles of Healthcare Epidemiology
Frances J. Boly
Victoria J. Fraser
Jennie H. Kwon
Epidemiology is defined as the study of the factors determining the occurrence of disease in human populations. Epidemiology is an indispensable tool for characterizing infectious diseases in medical institutions, communities, countries, and worldwide. Epidemiologic methods are necessary to determine the exposure-disease relationship and modes of acquisition and transmission that are critical for treatment, control, and prevention of infectious disease. Clinicians, microbiologists, and other personnel involved in public health and disease prevention professions use epidemiologic methods for disease surveillance, outbreak investigations, infectious diseases outcome measurements, and observational studies to identify risk factors for various infectious diseases. Knowledge of these risk factors is essential for making decisions regarding further epidemiologic or microbiological investigations, directing research activities, implementing relevant prevention and control measures or interventions, and establishing public health policies. In the pharmaceutical and biomedical industries, the application of epidemiologic methods is integral to the investigation of product contamination, ascertainment and characterization of risk factors for contamination, and maintenance of quality assurance practices in the laboratory or manufacturing operations to ensure safe distribution of products.
The use of epidemiology and the use of statistical methods to analyze epidemiologic data grew out of attempts to understand, predict, and control the great epidemics of the past; the diseases associated with those early epidemics were largely infectious. The study and implementation of infection control and prevention practices and interventions in hospitals were developed to understand and control the acquisition of infectious diseases that were initially described due to Staphylococcus aureus outbreaks in hospitals in the 1950s.1,2 Thus, discussion of the principles of epidemiology begins with examples of methods that were first formalized in the study of transmissible microorganisms, many of which continue to cause problems today.
The term hospital epidemiology was coined by professionals in the United States,3 as was the recognition of the use of epidemiologic methods for the study and control of noninfectious diseases in hospitals.4 The term nosocomial infection has traditionally defined acute infections acquired in hospital inpatient settings.5 However, in the current era of managed care, healthcare systems in the United States have evolved from traditional acute care hospital inpatient settings to more integrated, extended care models that now encompass hospitals, outpatient clinics, ambulatory centers, long-term care facilities, and the community. As expected, infections may be acquired at any of these levels of care. For this reason, the term nosocomial infection has been replaced by healthcare-associated infection (HAI).6
The terms hospital epidemiology and infection control and prevention remain synonymous in the minds of many, and both the terms and their associated programs have grown in definition and scope over the past five decades. With advances in the medical field and technology, interest in infection control and prevention has broadened from an initial focus on puerperal sepsis and surgical site infections to full, scientifically tested programs of surveillance, prevention, and control of all types of HAIs. Hospital epidemiology programs were among the first hospital disciplines to demonstrate the utility of the scientific method and statistics to characterize and analyze infectious diseases data and use the results of these analyses to improve the quality of healthcare and patient outcomes. In the special environment of the acute care hospital, a natural repetition of
earlier studies of population-based infectious disease outbreaks provided the basis for epidemiologic investigations.
earlier studies of population-based infectious disease outbreaks provided the basis for epidemiologic investigations.
Surveillance data generated from epidemiologic studies may be used to determine the need for clinical or public health action; assess the effectiveness of prevention, intervention, or control programs, or diagnostic algorithms; or set priorities for rational or appropriate use of limited microbiology resources, planning, and research. An understanding of epidemiology is important to quantify and interpret microbiology and pharmaceutical data and to apply these data to clinical practice, quality assurance, and hypothesis generation during investigation of outbreaks and other adverse events. Epidemiology is also an important foundation for rational medication prescribing policies, antimicrobial stewardship, and other public health measures.
Data from epidemiologic and microbiological studies can inform diagnostic and therapeutic practice and indicate areas for allocation of scarce resources. For example, one of the perennial problems that clinicians and microbiologists face is how to differentiate between a true bloodstream infection (BSI) and blood culture contamination resulting from coagulase-negative Staphylococci, which are the most frequently isolated microorganisms in blood cultures.7,8,9 Blood culture contamination can occur during venipuncture with inadequate antisepsis of the skin, after the blood draw at the time of inoculation of blood into the culture bottle, or during processing of blood culture bottles in the microbiology laboratory. To make an informed decision about whether a positive blood culture is a true BSI vs contamination, clinicians and microbiologists need to be familiar with the epidemiology of BSIs in different clinical settings and be able to integrate these data with relevant clinical and microbiology information so that a decision can be made whether or not to initiate antimicrobial therapy.
DEFINITIONS
In the application and discussion of epidemiologic principles, standard definitions and terminology have been widely accepted.10,11,12 The definitions of some commonly used terms are outlined in this section:
Attack rate: A ratio of the number of new infections divided by the number of exposed, susceptible individuals in a given period, usually expressed as a percentage. Other terms are the incidence rate and the case rate.
Attributable mortality: Indicates that an exposure was a contributory cause of or played an etiologic role leading to death.
Attributable risk: The measure of impact of a causative factor. The attributable risk establishes how much of the disease or infection is attributable to exposure to a specific risk factor. Attributable risk is a proportion where the numerator is the difference between the incidence in exposed and unexposed groups and the denominator is the incidence for the exposed group.
Bias: The difference between a true value of an epidemiologic measure and that which is estimated in a study. Bias may be random or systematic. There are three common types of bias: selection bias, information bias, and confounding. Selection bias is a distortion in the estimate of effect resulting from the manner in which parameters are selected for the study population. Information bias depends on the accuracy of the information collected. Unrecognized, systematic bias presents the greatest danger in studies by suggesting relationships that are not valid.
Carrier: An individual (host) who harbors a microorganism (agent) without evidence of disease and, in some cases, without evidence of host immune response. This carriage may take place during the latent phase of the incubation period as a part of asymptomatic disease or may be chronic following recovery from illness. Carriers may shed microorganisms into the environment intermittently or continuously, and this shedding may lead to transmission. Shedding and potential transmission may be increased by other factors affecting the host, including infection by another agent.
Case: An individual in a population or group recognized as having a particular disease or condition under investigation or study. This definition may not be the same as the clinical definition of a case.
Case-fatality rate: A ratio of the number of deaths from a specific disease divided by the number of cases of disease, expressed as a percentage.
Cluster: An aggregation of relatively uncommon events or disease in time and/or space in numbers that are believed to be greater than expected by chance alone.
Colonization: The recovery or identification of a microorganism at a body site or sites without any overt clinical signs of inflammation, infection, or immune reaction in the host at the time that the microorganism is isolated. Colonization may or may not be a precursor of infection. Colonization may be a form of carriage and is a potential source of transmission.
Communicability: The characteristic of a human pathogen that enables it to be transmitted from one person to another under natural conditions. Infections may be communicable or noncommunicable. Communicable infections may be endemic, epidemic, or pandemic.
Communicable period: The time in the natural history of an infection during which transmission to other susceptible hosts may take place.
Confounding: An illusory association between two factors when, in fact, there is no causal relationship between the two. The apparent association is caused by a third variable that is both a risk factor for the outcome or disease and is associated with but not a result of the exposure in question.
Contact: An exposed individual who might have been infected through transmission from another host or the environment.
Contagious: Having the potential for transmission of an infectious pathogen.
Contamination: The presence of an agent (eg, microorganism) on a surface or in a fluid or material, therefore, a potential source for transmission.
Cumulative incidence: The proportion of at-risk persons who become diseased during a specified period of time.
Endemic: The usual level or presence of an agent or disease in a defined population during a given period.
Epidemic: An unusual, higher-than-expected level of infection or disease by an agent in a defined population in a given period. This definition assumes previous knowledge of the usual, or endemic, levels.
Epidemic curve: A graphic representation of the distribution of defined cases by the time of onset of their disease.
Epidemic period: The time period over which the excess cases occur.
Hyperendemic: The level of an agent or disease that is consistently present at a high incidence and/or prevalence rate.
Immunity: The resistance of a host to a specific agent, characterized by measurable and protective surface or humoral antibody and by cell-mediated immune responses. Immunity may be the result of specific previous experience with the agent (wild infection), from transplacental transmission to the fetus, or from active or passive immunization to the agent. Immunity is relative and governed through genetic control. Immunity to some agents remains throughout life, whereas for others, it is short-lived, allowing repeat infections by the same agent. Immunity may be reduced in extremes of age, through disease, or through immunosuppressive therapy.
Immunity: cell-mediated vs humoral: Cell-mediated immune protection, largely related to specific T-lymphocytic activity, as opposed to humoral immunity, which is measured by the presence of specific immunoglobulins (antibodies) in surface body fluids or circulating in the noncellular components of blood. Antibodies are produced by B lymphocytes, also now recognized to be under the influence of T-lymphocytic function.
Immunogenicity: An agent’s (microorganism’s) intrinsic ability to trigger specific immunity in a host. Certain agents escape host defense mechanisms by intrinsic characteristics that fail to elicit a host immune response. Other agents evoke an immune response that initiates a disease process in the host that increases cellular damage and morbidity beyond the direct actions of the microorganism itself. These disease processes may continue beyond the presence of living microorganisms in the host.
Incidence: The ratio of the number of new infections or disease in a defined population in a given period to the number of individuals at risk in the population. “At risk” is frequently defined as the number of potentially exposed susceptible persons. Incidence is a measure of the transition from a nondiseased to a diseased state and is usually expressed as numbers of new cases per thousands (1000, 10 000, or 100 000) per year.
Incidence rate or density: Similar to the incidence but members of the at-risk population may be followed for different lengths of time. Thus, the denominator is the sum of each person’s time at risk (ie, total person-time of observation).
Incubation period: The period between exposure to an agent and the first appearance of evidence of disease in a susceptible host. Incubation periods are typical for specific agents and may be helpful in the diagnosis of unknown illness. Incubation periods may be modified by extremes of dose or by variations in host immune function. The first portion of the incubation period following colonization and infection is frequently a silent period, called the latent period. During this time, there is no evidence of host response(s) and evidence of the presence of the infecting agent may not be measurable. However, transmission of the microorganism to other hosts, though reduced during this period, is a recognized risk (eg, chicken pox, hepatitis B virus, human immunodeficiency virus [HIV]). Measurable early immune responses in the host may appear shortly before the first signs and symptoms of disease, marking the end of the latent period. Signs and symptoms of disease commonly appear shortly thereafter, marking the end of the incubation period.
Index case: The first case to be recognized in a series of transmissions of an agent in a host population. In semiclosed populations, as typified by chronic disease hospitals, the index case may first introduce an agent not previously active in the population.
Infection: The successful transmission of a microorganism to the host with subsequent multiplication, colonization, and invasion. Infection may be clinical or subclinical and may not produce identifiable disease. However, it is usually accompanied by measurable host response(s), either through the appearance of specific antibodies or through cell-mediated reaction(s) (eg, positive tuberculin test results). An infectious disease may be caused by the intrinsic properties of the agent (invasion and cell destruction, release of toxins) or by associated immune response in the host (cell-mediated destruction of infected cells, immune responses to host antigens similar to antigens in the agent).
Infectivity: The characteristic of the microorganism that indicates its ability to invade and multiply in the host. It is frequently expressed as the proportion of exposed patients who become infected.
Infestation: The presence of ectoparasites on the body (eg, scabies, lice).
Isolation: The physical separation of an infected or colonized host, including the individual’s contaminated body fluids and environmental materials, from the remainder of the at-risk population in an attempt to prevent transmission of the specific agent to the latter group. This is usually accomplished through individual environmentally controlled rooms or quarters, hand washing following contact with the infected host and environment, and the use of barrier protective devices, including gowns, gloves, and, in the case of airborne agents, an appropriate mask.
Morbidity rate: The ratio of the number of persons infected with a new clinical disease to the number of persons at risk in the population during a defined period, an incidence rate of disease.
Mortality rate: The ratio of those infected who have died in a given period to the number of individuals in the defined population. The rate may be crude, related to all causes, or disease-specific, related or attributable to a specific disease in a population at risk for the disease.
Odds: The ratio of the probability of an event occurring to the probability of it not occurring.
Pandemic: An epidemic that spreads over several countries or continents and affects many people.
Pathogenicity: The ability of an agent to cause disease in a susceptible host. The pathogenicity of a specific agent may be increased in a host with reduced defense mechanisms. For some agent-host interactions, the resultant disease is due to the effects of exaggerated or prolonged action of defense mechanisms of the host.
Prevalence: The ratio of the number of individuals measurably affected or diseased by an agent in a defined population at a particular point in time. The proportion of the population having the disease during a specified time period, without regard to when the process or disease began, defines the period prevalence.
Pseudo-outbreak: Real clustering of false infections or artifactual clustering of real infections. Often it is identified when there is increased recovery of unusual microorganisms.
Rate: An expression of the frequency with which an event occurs in a defined population. All rates are ratios. Some rates are proportions; that is, the numerator is a part of the denominator. A comparable rate is a rate that controls for variations in the distribution of major risk factors associated with an event.
Ratio: An expression of the relationship between a numerator and a denominator where the two are usually distinct and separate quantities, neither being a part of the other.
Relative risk: The ratio of the incidence rate of infection in the exposed group to the incidence rate in the unexposed group. Used to measure the strength of an association between exposures or risk factors and disease.
Reservoir: Any animate or inanimate niche in the environment in which an infectious agent may survive and multiply to become a source of transmission to a susceptible host. Medical care workers and patients constitute the main animate reservoir for microorganisms associated with HAIs; water-related reservoirs are important inanimate reservoirs that have been implicated in outbreaks related to dialysis units and to air conditioning systems.
Secular trend: Profile of the changes in measurable events or in the incidence rate of infection or disease over an extended period of time, also called a temporal trend.
Sensitivity: For surveillance systems, the ratio of the number of patients who were reported to have an infection divided by the number of patients who actually did have an infection (true positives over true positives plus false negatives).
Source: The animate or inanimate mechanism by which a pathogen is transmitted from their reservoir to a human (eg, the reservoir may be the soil, the source may be contaminated food). At times, the reservoir and source may be same (eg, sexually transmitted disease).
Specificity: For surveillance systems, the ratio of number of patients who were reported not to have an infection divided by the number of patients who actually did not have an infection (true negatives over true negatives plus false positives).
Sporadic: Occurring irregularly and usually infrequently over a period of time.
Surveillance: The ongoing systematic collection, analysis, and interpretation of healthcare data essential to the planning, implementation, and evaluation of public health practice, closely integrated with the timely dissemination of these data to those contributing data or to other interested groups who need to know the data. Surveillance was popularized by Langmuir and others at the Centers for Disease Control and Prevention (CDC) and has been an essential tool for infection control and prevention programs in the United States since the 1960s.
Susceptibility: A condition of the host that indicates absence of protection against infection by an infectious agent. This is usually marked by the absence of specific antibodies or specific measures of cell-mediated immunity against the infecting microorganism.
Transmission: The method by which any potentially infecting agent is spread to another host. Transmission may be direct or indirect. Direct transmission may take place by touching (direct contact transmission) between hosts, by the projection of large droplets in coughing and sneezing onto another host (droplet transmission), or by direct contact by a susceptible host with an environmental reservoir of the agent. Indirect transmission may be vehicle-borne, airborne, or vector-borne. In vehicle-borne transmission, contaminated environmental sources, including water, food, blood, and laundry, may act as an intermediate source of an infectious agent for introduction into a susceptible host. The infectious agent may have multiplied or undergone biologic development in the vehicle. In airborne transmission, aerosols containing small (1-5 µm) particles may be suspended in the air for long periods of time and then inhaled into the lower respiratory tract allowing replication of the infectious agent in a host. Airborne infectious particles may be generated by evaporation of larger particles produced in coughing and sneezing (Mycobacterium tuberculosis), by machines or equipment such as air conditioners or humidifiers that can generate aerosols (Legionella), or by wind or air currents (fungal spores). In vector-borne transmission, arthropods or other invertebrates may carry and subsequently transmit microorganisms, usually through inoculation of the host that occurs through biting the host or by contamination of food or other materials. The vector may be infected itself or only serve as mechanical carrier of the agent. If the vector is infected, the agent may have multiplied or undergone biologic development in the vector. Vector-borne transmission is an uncommon source of HAIs in the United States; however, vector-borne HAIs can occur in resource limited settings.
Virulence: The intrinsic capabilities of an agent to infect a host and produce disease and a measure of the severity of the disease produced. In the extreme, virulence is represented by the number of patients with clinical disease who develop severe illness or die, that is, the case-fatality rate.
EPIDEMIOLOGIC METHODS APPLIED TO INFECTIOUS DISEASES
Classic epidemiologic methods are essential for the study, characterization, and understanding of the various infections that occur in healthcare settings, communities, and broad geographic regions. Epidemiologic methods are used to determine the exposure-disease relationship in humans; establish the modes of acquisition, mechanisms of transmission, and spread; identify risk factors associated with infection and disease; characterize and relate causal factors to an infectious disease; determine or select appropriate methods of prevention and control; or guide rational application and practice of clinical microbiology methods. These epidemiologic methods were developed in an attempt to control common errors in observational studies
that occur when individuals study the association of one event (a risk or causal factor) with another later event (the outcome or disease).13,14,15,16
that occur when individuals study the association of one event (a risk or causal factor) with another later event (the outcome or disease).13,14,15,16
Epidemiologic study methods are grouped as either observational or experimental. Observational epidemiologic methods are further classified as either descriptive or analytic. Observational studies are conducted in natural, everyday community or clinical settings, where the investigators observe the appearance or an outcome but have no control over the environment or the exposure of people or product to a risk factor or suspected etiologic agent, a specific intervention or preventive measure, or a particular therapeutic regimen.
Descriptive Epidemiology
Observational descriptive studies establish the case definition of an infectious disease event by obtaining data for analysis from available primary (eg, medical records) or secondary (eg, infection control surveillance) sources. These data enable the characteristics of the population that has acquired the infection to be delineated according to (1) “person” (age, sex, race, marital status, personal habits, occupation, socioeconomic status, medical or surgical procedure or therapy, device use, underlying disease, or other exposures or events), (2) “place” (geographic occurrence of the health event or outbreak, medical or surgical service, place of acquisition of infection, or travel), and (3) “time” (preepidemic and postepidemic periods, seasonal variation, secular trends, or duration of stay in hospital). The information from descriptive studies might provide important clues regarding the risk factors associated with infection, and in each case, it is hoped that an analysis of the collected data might be used to generate hypotheses regarding the occurrence and distribution of disease or infection in the population(s) being studied.
Analytic Epidemiology
Observational analytic studies are designed to test hypotheses raised by the findings in descriptive investigations. The objectives of these studies are (1) to establish the cause and effects of infection in a population and (2) to determine why a population acquired a particular infection in the first place. The three most common types of observational analytic studies are cohort studies, case-control studies, and prevalence or cross-sectional studies.
Cohort Studies In cohort studies, hypotheses that have been generated from previous (descriptive) studies are tested in a new population. A population of individuals (a cohort) that is free of the infection or disease of interest is recruited for study. The presence or absence of the suspected (hypothesized) risk factors for the disease is recorded at the beginning of the study and throughout the observational period. All members of the cohort population (eg, all premature infants admitted to a neonatal intensive care unit [ICU] during a defined time period) are followed over time for evidence or appearance of the infection or disease and classified accordingly as exposed or unexposed to specific risk factors. If the observation period begins at the present time and continues into the future or until the appearance of disease, the study is called a prospective cohort study. If the population studied is one that in the past was apparently free of the markers of disease on examination of records or banked laboratory specimens, it may be chosen for study if data on exposure to the suspected risk factors for disease are also available. The population may be followed to the present or until the appearance of disease. This type of study, common in occupational epidemiology, is called a historical or retrospective cohort study.
A key requirement of a cohort study is that participants be reliably categorized into exposed and unexposed groups. Relative risk, that is, the ratio of the incidence of the outcome in the exposed group to the incidence in the unexposed group, is used to measure the strength of an association between exposures or risk factors and disease. Cohort studies have the advantage of enabling identification and direct measurement of risk factors associated with disease, determination of the incidence of infection and disease, and ascertainment of the temporal relationship between exposure and disease. In cohort studies, observational bias may be less of a limitation on the validity or results, since the information on the presence of risk factors is recorded before the outcome of disease is established. To ensure sufficient numbers for analysis, cohort studies require continual follow-up of large populations for long periods unless the disease under investigation is one of high incidence. Cohort studies are, in general, more expensive and time-consuming to conduct and are not suitable for the investigation of uncommon infections or conditions. However, they render the most convincing nonexperimental approach for establishing causation.
Case-Control Studies In a case-control study, individuals (cases) who are already infected, ill, or meet a given case definition are compared with a group of individuals (controls) who do not have the infection, disease, or other outcome of medical interest. In contrast to cohort studies, participants in a case-control study are selected by manifestation of symptoms and signs, laboratory parameters, or a specific condition, disease, or outcome. Thus, the search for exposure of case and control subjects to potential risk factors remains a retrospective one. For case-control studies, the measure of association between exposures or risk factors and health outcomes are expressed as an odds ratio, that is, the ratio of the odds of an exposure, event, or outcome occurring in a population to the odds in a control group, where the odds of an event is the ratio of the probability of it occurring to the probability of it not occurring.
The presence of significant differences in the exposure to risk factors among cases vs control subjects suggests an etiologic (causal) association between those factors and the infection or disease defined by cases. Case-control methods are useful for studying infections, events, or outcomes likely associated with multiple risk factors or low incidence rates; for investigating situations in which there is a long lag-time between exposure and outcome of interest; and for establishing etiologic associates or causation of a disease, infection, or other outcome when there is no existing information about the cause or source. In an attempt to reduce bias, control subjects might be selected from individuals matched with cases for selected characteristics, such as age, gender, socioeconomic status, or other variables not suspected or under investigation as risk factors. Compared with cohort studies, case-control studies may be conducted in relatively shorter time, are
relatively less expensive, or may require a smaller samples size to execute. Limitations of case-control studies include selection bias in choosing cases and control subjects; recall bias in which study subjects might have difficulty in remembering possible exposures; incomplete information on specific exposures; or risk factor data may be difficult to find (or remember). Case-control studies are not used to measure incidence or prevalence rates and, generally, are not capable of establishing temporal relationships between an exposure and outcome.
relatively less expensive, or may require a smaller samples size to execute. Limitations of case-control studies include selection bias in choosing cases and control subjects; recall bias in which study subjects might have difficulty in remembering possible exposures; incomplete information on specific exposures; or risk factor data may be difficult to find (or remember). Case-control studies are not used to measure incidence or prevalence rates and, generally, are not capable of establishing temporal relationships between an exposure and outcome.
Prevalence or Cross-sectional Studies In prevalence studies, the presence of putative risk factors and the disease under investigation is recorded in a survey of a study population at a specific point in time or within a (short) time period. The rates of disease among those with and without the suspected risk factors are compared. Thus, cross-sectional studies can establish association but not causation for suspected risk factors. Prevalence studies are relatively inexpensive and can be carried out rapidly if well planned. However, they do not allow the ascertainment of risk factors at the beginning of disease nor do they enable one to establish a temporal sequence of risk factors preceding the infection or other outcome of interest. Point prevalence, period prevalence, and seroprevalence surveys are examples of cross-sectional studies.
Experimental Epidemiology
In experimental studies, the investigator compares an exposure of individuals in a population to a suspected causal factor, a prevention measure, a therapeutic regimen, or some other specific intervention. These exposure modalities are randomly allocated to comparable groups, thereby minimizing confounding factors. Both the exposed and unexposed groups are monitored thereafter for specific outcomes (eg, appearance of infection or disease, evidence of effective prevention or control of the disease, or cure). Experimental studies often are used to evaluate antimicrobial or vaccine treatment regimens and are generally more expensive to conduct than observational studies. Within healthcare settings, studies that examine restriction of certain antimicrobials or promotion of use of alternative antimicrobials for the control of antimicrobial resistance could be considered under the category of experimental. For ethical reasons, it is rarely possible to expose human populations to potential pathogens or to withhold a preventive measure that could potentially be beneficial to patients. Unfortunately, many animal hosts are not naturally susceptible to many agents of human disease. Thus, one has to be careful when extrapolating epidemiologic findings in animal experimental studies to the control of infections in human subjects.
Quasi-experimental studies: This type of study shares the design characteristics of experimental studies but lacks random assignments of study subjects. Quasi-experimental studies are useful where randomization is impossible, impractical, or unethical. The main drawbacks of quasi-experimental studies are their inability to eliminate confounding bias or establish causal relationships. The most common quasi-experimental design is the beforeafter study in which there is a baseline measurement of the outcome (prospectively or retrospectively) followed by an intervention with measurement of the outcome and an assessment whether the intervention altered the outcome.
Machine Learning
In the United States, the implementation and integration of electronic medical records into many inpatient and outpatient healthcare settings has led to an unprecedented quantity of healthcare data. Electronic health record (EHR) data can be used to improve infection control and prevention and allocation of hospital resources. Machine learning is the study of tools and methods for identifying patterns in data. It is becoming increasingly used for healthcare projects for risk stratification for specific infections, identifying the relative contribution of specific risk factors to overall risk, understanding pathogen-host interactions, and predicting emergence and spread of infectious diseases.17 Machine learning can be divided into two subareas: supervised and unsupervised learning. In supervised learning, the input data and target outcomes are observed. An example is classifying patients as either diseased or healthy. A domain expert (eg, a physician) assigns a label to each patient. The aim being to find a model that best distinguishes between the two classes, to either correctly assign the label or new patients or to identify important covariables. In unsupervised learning, the data consist of input variables without a corresponding outcome label. The goal is to find patterns and extract hidden data without expert labeling.18 An example of unsupervised learning is detection of patients with hospital-acquired infections and finding similar patient subgroups to assign patients to clinical trials. Numerous studies have incorporated machine learning to the field of infectious diseases including predicting the risk of nosocomial Clostridioides difficile infection (CDI) using clinical data from the EHR, prediction of central line-associated bloodstream infections (CLABSI), predicting reservoirs of zoonotic diseases using information on rodent species, and predicting clinical outcomes in Ebola virus using clinical symptoms.17 There are numerous challenges to applying machine learning for the purposes of hospital epidemiology. Given the potential inconsistencies and inaccuracies of health data, significant time can be required to extract and reprocess data before it can be used for machine learning. Machine learning with big data is not a replacement for a randomized control trial. No matter how detailed the measurements in EHR data, unmeasured factors cannot always be captured and observational studies of EHR data lack the ability to identify causal inference.19
EPIDEMIOLOGY OF INFECTION AND DISEASE
The epidemiology of infectious disease presents two processes for discussion: (1) the epidemiology of the determinants leading to infections in hosts and (2) the epidemiology of the appearance and extent of disease related to the infection in those hosts. It is common to discuss health and disease as the result of a series of complex interactions between an infectious agent, the host that is the target of the infectious agent’s actions, and the mutual environment in which the host and infectious agent exist. In studies of HAIs, the infectious agents are the microorganisms associated
with the infections, the hosts are the patients or healthcare personnel (HCP), and the common environment is the acute care hospital, ICU, outpatient, home, or other healthcare setting.
with the infections, the hosts are the patients or healthcare personnel (HCP), and the common environment is the acute care hospital, ICU, outpatient, home, or other healthcare setting.
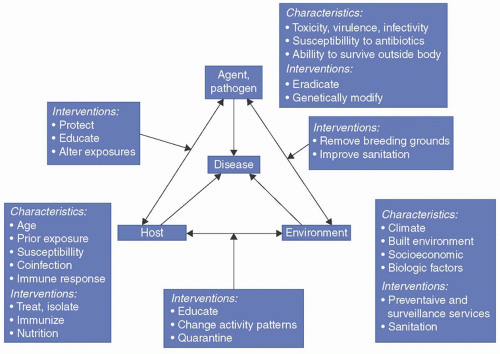
FIGURE 1-1 The “epidemiologic triad” of infectious disease summarizes the factors that influence an infection and the measures you might take to combat the infection. (From Johnson Y, Kaneene J. Epidemiology: From Recognition to Results. 2018:1-30. Ref.21)
|
The interactions determining the probability of a microbiologic agent causing infection in a host may be simply presented by an equation of infection:
where Ip is the probability of infection, D is the dose (number of microorganisms) transmitted to the host, S is the receptive host site of contact with the agent, T is the time of contact (sufficient for attachment and multiplication or not), and V represents virulence, the intrinsic characteristics of microorganism that allow it to infect. The denominator in the equation (Hd′) represents the force of the combined host defenses attempting to prevent the infection.
Any reduction in host defenses (represented by the denominator) in such an equation allows infection to take place with a similar reduction in one or more of the infectious agent factors in the numerator. Infection may take place with a smaller dose of microorganisms. Infection may take place at an unusual site. The contact time for a microorganism to attach to an appropriate surface may be shorter, or infection may take place with an agent of lesser virulence, one that does not cause infection in a normal host. Reductions in the host defense characteristics, represented by the denominator, and the reduction of requirements to infect for the agent are typical of the interactions that facilitate opportunistic infections in compromised hosts, increasing numbers of whom receive medical and surgical care in modern hospitals. In the equation of infection, the environment might be considered the background on which the infectious agent-host interaction takes place.20 A number of additional models of the interaction of agent, host, and environment have been suggested to help understand these processes. The model in Figure 1-1, the triangle model, attempts to visualize the interplay between the three components of the host pathogen and environment.
INTERACTIONS OF AGENT, HOST, AND ENVIRONMENT
There are often multifactorial causes that lead to outcome events (infection or disease). For some infectious diseases, a single unique factor or agent is necessary and sufficient for the disease to appear. This is exemplified by measles or rabies. It is only necessary for the host to be exposed to and infected by an agent (the measles virus or rabies virus) for that disease to develop. For other infectious diseases, the single factor of infectivity of the agent is necessary but not sufficient to cause disease in the host. Mycobacterium tuberculosis (M tuberculosis), polio virus, hepatitis A, and many other agents necessary for specific disease in a human host can infect hosts without causing disease in many cases. Within the hospital setting, exposure to a specific microorganism or colonization of an inpatient with an agent, such as vancomycin-resistant enterococci (VRE) or S aureus, may be necessary but not sufficient to generate disease. For VRE and S aureus, disease generally develops as a result of complex interactions between contributing factors, such as age, state of debilitation, immune or nutritional status, device use, invasive procedures, antimicrobial usage, or susceptibility of the microorganism to available antimicrobials. The presence of the infection in these cases is not always sufficient to produce disease in the host without the contribution of additional host or environmental factors.
Agents
The agents causing healthcare-associated infectious diseases are diverse microorganism ranging in size and
complexity from viruses and bacteria to protozoa and helminths. Bacteria, fungi, and certain viruses have been the most recognized and studied causes of HAIs.22 For transmission to take place, microorganisms must remain viable in the environment until contact with the host has been sufficient to allow infection. Reservoirs that allow the agent to survive or multiply may be animate, as exemplified by HCP carriage of S aureus in the anterior nares or throat,23 or inanimate as occurs in the environment, as demonstrated by Pseudomonas spp. colonization of sink areas, Legionella in hot and cold water supply systems,24 C difficile spores on computer keyboards,25 or Serratia marcescens growing in contaminated soap or hand lotion preparations.26
complexity from viruses and bacteria to protozoa and helminths. Bacteria, fungi, and certain viruses have been the most recognized and studied causes of HAIs.22 For transmission to take place, microorganisms must remain viable in the environment until contact with the host has been sufficient to allow infection. Reservoirs that allow the agent to survive or multiply may be animate, as exemplified by HCP carriage of S aureus in the anterior nares or throat,23 or inanimate as occurs in the environment, as demonstrated by Pseudomonas spp. colonization of sink areas, Legionella in hot and cold water supply systems,24 C difficile spores on computer keyboards,25 or Serratia marcescens growing in contaminated soap or hand lotion preparations.26
Certain intrinsic and genetically determined properties of microorganisms are important for their survival in the environment. Intrinsic and genetic properties that enhance survival include the ability to resist the effects of heat, drying, ultraviolet light, or chemical agents, especially antimicrobials, and the ability to independently multiply in the environment or to develop and multiply within another host or vector. Intrinsic infectious agent factors important to the production of disease include infectivity; pathogenicity; virulence; the infecting dose; the infectious agent’s ability to produce toxins; its immunogenicity and ability to resist or overcome the human immune defense system; its ability to replicate only in certain types of cells, tissues, or hosts (vectors); its ability to persist or cause chronic infection; and its interaction with other host mechanisms, including the ability to cause immunosuppression (eg, HIV).
Once transferred to a host surface, infectious agents may multiply and colonize without invading or evoking a measurable host immune response.27 The presence of an infectious agent at surface sites in the host does not define the presence of an infection. Nonetheless, colonized patients may act as the reservoir and/or source of transmission to other patients or HCP.28
If infection takes place, a measurable immune response will develop in most hosts even if the infection is subclinical. The likelihood of infectious agents causing infections is increased in nonimmune hosts, and infections are most likely to occur in nonimmune, immunocompromised hosts. A microorganism’s ability to infect another host vector (eg, yellow fever virus in mosquitoes) or another nonhuman reservoir (eg, yellow fever virus in monkeys) is important in the epidemiology of certain infectious diseases globally but plays little role in the epidemiology of HAIs where vector-borne infectious diseases are uncommon and where hospital settings have sealed windows/doors and controlled heating, ventilation, and air conditioning (HVAC) systems in the United States.
Host
Infection depends on exposure of a susceptible host to an infecting agent. Exposure of the susceptible host to such agents is influenced by age, behavior, family associations, occupation, socioeconomic level, travel, avocation, access to preventive healthcare, vaccination status, or hospitalization. Whether or not disease takes place in the infected host and the severity of disease when it occurs depends not only on the intrinsic virulence factors of the infectious agent but also more importantly on the pathogenicity of the infectious agent and host pathogen interactions. The host immune defenses exist to prevent infection. Thus, any reduction in host defenses may allow infection to take place with a smaller dose of microorganisms or at a body site that is not usually susceptible to infection. A combination of reductions in host defense characteristics and the requirements for an agent to cause infection are typical of the interactions that allow acquisition of opportunistic infections in immunocompromised patients.29,30
Host factors important to the development and severity of infection or disease may be categorized as intrinsic or extrinsic. Intrinsic factors include the person’s age at infection; birth weight; sex; race; nutritional status31; comorbid conditions (including anatomic anomalies) and diseases; genetically determined immune function; immunosuppression associated with other infections, diseases, or therapy; vaccination or immunization status; previous exposure or infection with an infectious agent or similar agents; and the psychological state of the host.32,33 Colonization of the upper and lower respiratory tracts is more likely when the severity of illness increases in critically ill patients. This, along with other host impairments (eg, reduced mucociliary clearance or changes in systemic (pH), allows colonization to progress to invasive infection. Moreover, other clinical conditions may lead to an alteration in epithelial cell surface susceptibility to binding by bacteria, leading to enhanced colonization.27 Extrinsic factors include invasive medical or surgical procedures, the presence of medical devices, such as intravenous catheters or mechanical ventilators, specific behaviors such as sexual practices and contraception, duration of antimicrobial therapy and hospitalization, and exposure to hospital personnel.
Environment
The environment serves as the background on which infectious agent-host interactions take place. Environmental factors can positively or negatively influence the spread of infection. Environmental factors that influence the spread of infection include (1) physical factors such as climate conditions of heat, cold, humidity, seasons, and the built environment (eg, ICUs, outpatient clinics, long-term care [LTC] facilities, or water reservoirs); (2) biologic factors (eg, the presence or absence of intermediary hosts such as insect or snail vectors, presence of biofilms in sinks or devices); and (3) social factors (eg, socioeconomic status, types of food and methods of preparation, and availability of adequate housing, potable water, adequate waste disposal, and healthcare amenities).34 These environmental factors influence both the survival and the multiplication of infectious disease agents in their reservoirs and the behavior of the host in housing, occupation, and recreation that relate to exposure to pathogens. Food- and waterborne diseases flourish in warmer months due to better incubation temperatures for microorganism multiplication and the recreational exposures of the host, whereas respiratory agents appear to benefit from increased opportunities for airborne and droplet transmission in the reduced humidity and closer living environments during the winter. In U.S. hospitals, the frequency of hospital-acquired Acinetobacter spp. infections is increasing in ICUs. The seasonal variation in the incidence of Acinetobacter spp. infections is thought to be due to changes in climate since the number of Acinetobacter spp. infections identified increases in the summer months.35
Within healthcare settings, the components of the agent, host, and environment triad interact in a variety of ways to produce HAIs. For example, ICUs are now considered the areas with the highest risk for the transmission of healthcare-associated pathogens in U.S. hospitals.36,37 Moreover, methicillin-resistant S aureus (MRSA), VRE, extended spectrum β-lactamase (ESBL) producing E coli and Klebsiella, and β-lactam-resistant Pseudomonas aeruginosa are endemic in many ICUs in these hospitals.36,37 The emergence of vancomycin-resistant S aureus (VRSA) in U.S. institutions in 2002 highlighted the unwelcome but inevitable reality that VRSA may become endemic in acute care settings.38,39 A complex interaction of contributory factors, such as inadequate hand hygiene and suboptimal infection control practices among HCP, fluctuating staffing levels, an unexpected increase in patient census relative to staffing levels in the ICU, or an unprecedented increase in the number of severely ill patients with multiple invasive devices, could all contribute to the acquisition of hospital or long-term care facility infections caused by one of these endemic microorganisms.40,41,42,43,44 Adding to the complexity of the process would be the unquantifiable mechanism of transmission of the agent from the host to HCP, HCPto-HCP, and host-to-environment. Thus, acceptable measures for the prevention and control of HAIs dictate that healthcare epidemiologists study and analyze the interrelationships among all components of the triad of agent, host, and environment.36,37
It is well known that the social environment is important in influencing personal behavior that affects the direct transmission of agents, such as HIV transmission to infants from nursing mothers via breast milk in regions of high HIV endemicity, Gram-negative microorganisms transmitted via artificial nails worn by HCP in U.S. ICUs,43,45 and increased risk of sexually transmitted infections among vulnerable populations who cannot always use condoms or other barrier protection or do not have access to preexposure prophylaxis. What must be understood to be equally relevant is the impact of other factors in the social environment, such as the distribution of and access to medical resources; the use of preventive services46,47,48,49,50; the enforcement of codes in food preparation, infection prevention practices, or occupational health practices; the extent of acceptance of breast-feeding for children51,52,53; and the acceptance of advice on the appropriate use of antimicrobials.54,55,56,57,58 Also, there must be an appreciation by patients, relatives, and healthcare providers alike that at-risk patients (eg, those born very prematurely, the very elderly, or those with premorbid end-stage cardiac or pulmonary disease) who have numerous indwelling catheters or invasive devices, or who have undergone multiple invasive or surgical procedures will be particularly susceptible to HAIs that are likely nonpreventable.
Special Environments
Microenvironments, including military barracks, dormitories, day care centers, LTC settings, ambulatory surgery and dialysis centers, and acute care hospitals, provide special venues for infectious agent-host interactions. Historically, epidemics in these institutional environments provided the experience that drove the development and acceptance of infection control and prevention measures, guidelines, and the need for formal infection control resources and infrastructure. Acute care hospitals, especially those offering regional, secondary, and tertiary care, remain the dominant examples of high-risk environments. Changing patterns of outpatient practice, home healthcare, and technical advances in medicine have resulted in increasingly severely diseased and injured populations being managed in acute care facilities. Data from the CDC demonstrate that the changing healthcare environments in the United States are resulting in increasing numbers of ICU patients, while there has been a general decrease in the number of general medical beds.22
Special units for intensive medical or surgical care for extensive burns, trauma, transplantation, and cancer chemotherapy frequently care for patients with increased susceptibility to infection.59 In these susceptible patients, reduced inocula of pathogens or commensals are required to cause infection, infection may take place at unusual sites, and usually nonpathogenic agents may cause serious disease and death. Frequent opportunistic infections in these patients often require repeated, broad-spectrum, and extended therapy with multiple antimicrobials, leading to increasingly resistant resident microbial populations.22,57,58 The emergence in ICU settings of pathogens resistant to all available antimicrobials is becoming more common.36 For example, P aeruginosa has the capability to acquire new resistance mechanisms through horizontal transfer or chromosome mutations. This can occur while the patient is actively being treated and result in antibiotic failure and lead to mortality.60 Additionally, VRE colonization and contamination in the ICU has been associated with transmission to other patients.61
Outpatient parenteral antimicrobial therapy (OPAT) was originally developed in the 1970s to treat cystic fibrosis patients. OPAT gives patients the opportunity to have central venous catheters (CVC) placed and kept in situ for intravenous home infusion therapy to avoid prolonged hospital stays. The Infectious Diseases Society of America (IDSA) has published guidelines listing requirements for teamwork, communication, monitoring, and outcome measurements in order to establish an effective and safe OPAT program.62,63 The benefit of OPAT is reduced exposure to hospital environments and decreased costs to patients, hospitals, and payers. OPAT also offers significant benefits to patients. It reduces their hospital stays and allows them to be in the familiar setting of their own home. Patients also learn to become advocates for their own health and ensure that appropriate and complete treatment is provided. On the other hand, a patient with a CVC in the home environment may potentially be at risk of CLABSI due to contamination of lines, dressings, and infusates in a care environment where infection control practices are not as well understood, practiced, or regulated. Some studies have reported readmission rates above 20% in patients participating in OPAT because of worsening infection, adverse drug reactions, and catheter-related complications. Recent studies have suggested that early infectious disease follow-up was associated with a lower 30-day hospital readmission rate.64
INFECTION, COLONIZATION, AND SPECTRUM OF DISEASE
Infection is the successful transmission of a microorganism to a susceptible host, through a suitable portal of entry, with subsequent colonization, multiplication, and invasion. The source of a microorganism (the primary reservoir) may be animate (eg, humans, mammals, reptiles, or arthropods) or inanimate (eg, work surfaces, toys, false fingernails, toiletries, or soap). Disease is the overt damage done to a host as a result of its interaction with the infectious agent; it represents a clinically apparent response by or injury to the host after infection, with the affected person showing symptoms or physical signs that may be characteristic of infection with the invading pathogen. Thus, disease is the outcome of an infectious process, and a pathogen is any microorganism with the capacity to cause disease in a specific host.
Unapparent or subclinical infection is a frequent occurrence in which the infected person may not manifest any symptoms, signs, disability, or identifiable disease. For example, subclinical Plasmodium falciparum infections in patients from Bangladesh can serve as a reservoir for malaria infection.65 In patients who acquire Salmonella typhi infection (typhoid fever), a chronic infection of the gallbladder may develop with asymptomatic fecal excretion of the pathogen for years after the acute infection.66 Patients in HIV-endemic countries may have M tuberculosis BSIs despite having normal chest radiographs and no symptoms or signs suggestive of underlying pulmonary disease.67,68 Persons with subclinical infection are sometimes referred to as carriers. Subclinical infection may be recognized through laboratory testing of blood or other appropriate body material from the host. These tests may indicate evidence of an immune response to infection, the presence of antigens characteristic of the microorganism, abnormal cellular function in response to infection, or the presence of the microorganism itself.
Colonization is the presence of a microorganism in or on a host, with growth and multiplication, but without any overt clinical expression or detected immune reaction in the host at the time the microorganism is isolated. An infectious agent may establish itself as part of a patient’s flora or may cause low-grade chronic disease after an acute infection. For example, 20% of healthy adults are persistent carriers of S aureus in the anterior nares without any manifestation of clinical illness. However, under suitable conditions, patient populations colonized with S aureus are at an increased risk of developing infections and disease.69,70,71,72 Once colonization or infection is established in a susceptible host, the infectious agent may enter a silent or latent period during which there is no clinical or typical laboratory evidence of its presence. Thereafter, the host may manifest signs and symptoms of mild disease without disability, exhibit rapid or slow progression of disease, or progress to either temporary or chronic disability. Ultimately, the patient may die or have a complete recovery and return to health without sequelae.
The outcome of an infection is determined by the size of the infecting dose, the site of the infection, the vaccination status of the host, the speed and effectiveness of the host immune response, other intrinsic host factors (eg, nutritional status), and promptness of instituting and effectiveness of the therapy. These factors together with intrinsic properties of a microorganism, such as its infectivity, pathogenicity, virulence, and incubation period, determine the course and progress of an infection and manifestation of disease. Infectivity is the characteristic of the microorganism that indicates its ability to invade and multiply in a susceptible host to produce infection or disease. Infectivity is expressed as the proportion (ie, the attack rate) of patients who become infected when exposed to an infectious agent. The basic measure of infectivity is the minimum number of infectious particles required to establish infection. Pathogens like Ebola, polio, or measles viruses have high infectivity.
The pathogenicity of an infectious agent is a measure of its ability to cause disease in a susceptible host. Thus, while the measles virus has a relatively high pathogenicity (ie, few subclinical cases), the poliovirus has a low pathogenicity (ie, most cases of polio are subclinical). The measure of pathogenicity is the proportion of infected persons with clinically apparent disease. The pathogenicity of an infectious agent that is usually innocuous may be increased in a host with reduced defense mechanisms. For some infectious agent-host interactions, the resultant disease is due to the effects of exaggerated or prolonged host defense mechanisms. The virulence of a microorganism is its intrinsic capability of infecting a host to produce disease. It follows that a pathogen might have varying degrees of virulence. Thus, although the nonencapsulated form of Haemophilus influenzae is a common inhabitant of the upper respiratory tract of healthy humans and causes localized infection without bacteremia (eg, conjunctivitis or otitis media in children), the more virulent encapsulated type b form causes more invasive disease and is an important cause of meningitis or epiglottitis. The incidence of H influenzae infection in the United States has dramatically decreased with the introduction of the H influenzae b vaccine. If the disease is fatal, virulence can be measured with the case-fatality rate. For example, the rabies virus almost always produces fatal disease in humans and is therefore an extremely virulent agent.
The ability to diagnose an infection or disease depends on the degree to which typical symptoms and physical signs develop in patients, the appropriateness of diagnostic tests, and the sensitivity and specificity of these tests for the particular infecting agent. Whether an infecting agent produces clinical or subclinical infections depends on the agent and host factors, for example, age or immune status. For example, P aeruginosa, a ubiquitous pathogen that thrives in aquatic environments and vegetation, seldom causes disease in healthy humans. However, in debilitated, hospitalized patients, such as those with burns, critical care patients with multiple invasive medical devices, or those who are on prolonged mechanical ventilation, P aeruginosa has become an important cause of HAIs in U.S. hospitals.73,74,75,76
Certain agents may be associated with a variety of different syndromes that depend on age and vaccination status of the host, previous infection with the agent, and agent-related mechanisms that remain unclear. Thus, Strongyloides spp., a nematode that is endemic in many parts of the world, including Southeast Asia and some
parts in southeastern United States, can cause asymptomatic infection or be associated with several syndromes ranging from mild epigastric discomfort and chronic skin rashes to life-threatening hyperinfection that results in a Gram-negative BSI, pneumonia, and multisystem disease in immunosuppressed patients, including solid organ transplant recipients or patients with chronic airway disease who are steroid-dependent.77,78,79 These differences in host-agent interactions underscore the difficulty in establishing causation and the importance of confirmatory laboratory evidence to precisely identify the causal agent associated with infectious disease syndromes.
parts in southeastern United States, can cause asymptomatic infection or be associated with several syndromes ranging from mild epigastric discomfort and chronic skin rashes to life-threatening hyperinfection that results in a Gram-negative BSI, pneumonia, and multisystem disease in immunosuppressed patients, including solid organ transplant recipients or patients with chronic airway disease who are steroid-dependent.77,78,79 These differences in host-agent interactions underscore the difficulty in establishing causation and the importance of confirmatory laboratory evidence to precisely identify the causal agent associated with infectious disease syndromes.
Once colonization or infection is established in a susceptible host, the agent may enter a silent or latent period during which there is no clinical or usual laboratory evidence of its presence. Thereafter, the host may manifest signs and symptoms of mild disease without disability, may have a rapid or slow progression of disease, or may progress to either temporary or chronic disability, or, ultimately, death. Alternatively, the patient may have a complete recovery and return to health without sequelae. In other instances, the entire process may be unapparent or subclinical without evidence of disability or disease. Subclinical cases may be recognized through laboratory testing of blood or other body fluids of the host. These tests may indicate evidence of abnormal cellular function (abnormal liver function tests), the presence of an immune response to infection (antibody to hepatitis B virus core antigen), the presence of antigens characteristic of the microorganism (positive test for hepatitis B virus surface antigen), or the presence of the microorganism itself.
The ability to diagnose an infection or disease is obviously easier in clinical cases and much easier in severe clinical cases wherein the typical signs and symptoms of the disease are apparent and routine tests are diagnostic of the agent. The ratio of clinical to subclinical infections varies widely by agent and is influenced by certain host factors, such as age, and immune status. Certain agents may be associated with a variety of different syndromes that depend on age and vaccination status of the host, previous infection with the agent, and agent-related mechanisms that remain unclear. Pertussis is often a severe disease in neonates but usually causes a three to six month mild respiratory infection in adults, and coxsackievirus B infections may appear as myocarditis 1 year and more prominently as meningoencephalitis the next. Respiratory syncytial virus infections may appear as bronchiolitis in infants and as a common cold syndrome in their older caregivers. Since the ability to diagnose an infection or disease caused by a specific pathogen depends partly on the degree to which typical symptoms and physical signs develop in patients, variation in the clinical manifestation of disease underscores the difficulty in establishing causation, the importance of clinical awareness of syndromic variations of certain infections, and the importance of confirmatory laboratory evidence to precisely identify the causal agent associated with syndromes of disease outbreaks. Evans provides a detailed and excellent review of the principles and issues in establishing causation in infection and disease.80,81,82
MECHANISM OF SPREAD
Transmission
For infection to take place, microorganisms must be transferred from a reservoir to an acceptable entry site on a susceptible host in sufficient numbers (the infecting dose) for multiplication to occur. The infecting dose of a microorganism may depend in varying degrees on infectivity, pathogenicity, or virulence of the microorganism itself. The entire transmission process constitutes the chain of infection. Within the healthcare setting, the reservoir of an agent may include patients themselves, HCP (eg, nares or fingernails), potable water, soap dispensers, hand lotions, mechanical ventilators, intravascular devices, infusates, multi-dose vials, or various other seemingly innocuous elements in the environment.
Direct transmission from another host (healthy or ill) or from an environmental reservoir or surface by direct contact or direct large-droplet spread of infectious secretions is the simplest route of agent spread. Examples of direct contact transmission routes include kissing (infectious mononucleosis), shaking hands (common cold [rhinovirus]), or other skin contact (eg, contamination of a wound with Staphylococcus spp. or Enterococcus spp. during trauma, surgical procedures, or dressing changes). Transmission of Neisseria meningitidis, group A Streptococcus, or the respiratory syncytial virus (an important cause of respiratory infection in young children worldwide) by large respiratory droplets that travel only a few feet is regarded as a special case of direct contact transmission.
Vertical transmission of infection from mother to fetus is another form of direct contact transmission that may occur through the placenta during pregnancy (eg, HIV, rubella virus, hepatitis B virus, or parvovirus) by direct contact of the infant with the birth canal during childbirth (group B streptococci) or via breast milk (HIV).
Indirect contact transmission may occur via the hands of people, contaminated inanimate objects (fomites), various work surfaces, food, biological fluids (eg, respiratory, salivary, gastrointestinal, or genital secretions, blood, urine, and stool), invasive or shared medical devices, or through arthropod or animal vectors. Indirect contact transmission is the most common mechanism of transfer of the microorganisms that cause HAIs and commonly occurs via the hands of HCPs, their clothing, or instruments like stethoscopes or thermometers. Rapid dissemination of agents, such as respiratory syncytial virus or the influenza virus may occur in day care centers through salivary contamination of shared toys and games. C difficile is an important cause of infectious diarrhea transmitted from patient to patient in acute care hospitals. Its transmission is abetted by its spore-forming ability to survive in the environment, and its selection and promotion in patients by the repeated and prolonged use of antimicrobials.83,84,85 Medical devices contaminated with bloodborne pathogens, including hepatitis B and C viruses and HIV, are sources of infection for both patients and HCPs in healthcare institutions. Some viruses can remain viable for extended periods under suitable conditions. For example, hepatitis B virus is relatively stable in the environment and remains viable in dried form for at least 7 days to 2 weeks on normal working surfaces
at room temperature. This property has led to hepatitis B virus transmission among dialysis patients through indirect contact via dialysis personnel or work surfaces in the dialysis unit.86,87,88,89,90,91 Examples of other sources of HAIs that occur through indirect contact include bacterial or viral contamination of musculoskeletal allograft tissues, intrinsic contamination of infusates or injectable medications, liquid soap dispensers, or contaminated medications prepared in the hospital pharmacy.92,93,94,95,96,97 The continuing presence of Pseudomonas spp. and other Gram-negative rods in potable water supplies acts as an important reservoir for these agents and a readily available source for hand transmission to patients, especially the severely ill.98,99,100,101,102
at room temperature. This property has led to hepatitis B virus transmission among dialysis patients through indirect contact via dialysis personnel or work surfaces in the dialysis unit.86,87,88,89,90,91 Examples of other sources of HAIs that occur through indirect contact include bacterial or viral contamination of musculoskeletal allograft tissues, intrinsic contamination of infusates or injectable medications, liquid soap dispensers, or contaminated medications prepared in the hospital pharmacy.92,93,94,95,96,97 The continuing presence of Pseudomonas spp. and other Gram-negative rods in potable water supplies acts as an important reservoir for these agents and a readily available source for hand transmission to patients, especially the severely ill.98,99,100,101,102
Airborne transmission is another mechanism of indirect transfer of pathogens. Microorganisms transmitted by this method include droplet nuclei (1-10 µm) that remain suspended in air for long periods, spores, and shed microorganisms. The airborne transfer of droplet nuclei is the principal route of transmission of M tuberculosis, varicella, and measles. The transmission of Legionella spp. through the air in droplet nuclei from cooling tower emissions, and from environmental water sites, such as air conditioning systems, central humidifiers, and respiratory humidification devices, is another important example of this type of spread.103,104,105,106,107,108 More recently, outbreaks of Mycobacterium chimaera were found to be secondary to contamination of exhaust air from heater-cooler devices used during cardiac surgery.109,110 The emergence of extensively drug-resistant (XDR) strains of M tuberculosis (ie, strains resistant to practically all second-line agents) has again highlighted the importance of airborne transmission and the fact that the underlying reason for XDR emergence stems from poor general tuberculosis control measures and the subsequent development of multidrug-resistant (MDR) tuberculosis.111,112,113
CDI, the most common cause of healthcare-associated diarrhea in the United States, can be acquired through the transmission of spores via hospital work surfaces and the hands of HCP.85,114,115 Fungal spores can be an important cause of HAIs. Spores of invasive fungi, such as Aspergillus spp., may be carried over long distances in hospitals to cause severe infections in immunosuppressed patients. The risk of spore contamination was highlighted by an outbreak of Curvularia lunata (a black fungus) among silicone breast implant recipients, who had undergone the breast augmentation procedures in an operating room that was erroneously maintained at negative pressure resulting in high spore counts in the operating room environment (operating rooms are supposed to be maintained at net positive pressures relative to adjacent areas).116 The surgeons had not implemented a closed system for inflating the breast prostheses with saline; instead, they had inflated the silicone prostheses using syringes filled with saline drawn up from a sterile bowl exposed to the ambient operating room environment. The end result was contamination of sterile saline in the open bowl with C lunata spores, which were then injected inadvertently into the breast prostheses.117 In some settings (eg, burn units), staphylococci have been thought to spread on skin squamous cells that have been shed from patients or HCP. The importance of this mode of transmission, however, is not thought to be of great significance in other care settings. More recent data suggest that S aureus is a common isolate in oropharyngeal cultures.118,119 Although the epidemiologic implications of this finding remain uncharacterized, the ramification for infection control in healthcare facilities would be enormous if indeed the chain of infection for S aureus includes oropharyngeal secretions or droplet nuclei.
Vector-borne transmission by arthropods or other insects is a form of indirect transmission and may be mechanical or biologic. In mechanical vector-borne transmission, the agent does not multiply or undergo physiologic changes in the vector; in biologic vector-borne transmission, the agent is modified within the host before being transmitted. Although the potential for microorganism carriage by arthropods or other insect vectors has been described,120,121 this type of transmission has not played any substantial role in the transmission of HAIs in the United States. In tropical countries with endemic dengue, yellow fever, or malaria, vector-borne transmission of infectious diseases is more important, requiring screening of patients or other interventions, and preventive measures not ordinarily required for patients in colder climates.
Reservoirs
Humans are the primary reservoir for Neisseria gonorrhoeae, Salmonella enterica serotype Typhi, HIV, hepatitis B and C viruses, and Shigella spp. Animals (zoonoses) harbor rabies virus, Yersinia pestis, Leptospira spp., nontyphi Salmonella, and Brucella spp. Environmental reservoirs include the soil (Histoplasma capsulatum, Clostridium tetani, and Bacillus anthracis) and water (Legionella spp., P aeruginosa, Serratia spp., and Acinetobacter spp). For some infections, the interaction between host, agent, and environment might include an extrinsic life cycle of the agent outside of the human host. The interplay of such factors can add significant layers of epidemiological complexity to properly understand the cause of an outbreak or in characterizing the chain of infection.
INCUBATION PERIOD AND COMMUNICABILITY
The incubation period is the time between exposure to an infectious agent and the first appearance of evidence of disease in a susceptible host. The incubation period of a pathogen usually is typical for that class of microorganisms and may be helpful in diagnosing unknown illness or making a decision regarding further diagnostic testing. The first portion of the incubation period after colonization and infection of a person is frequently a silent period, called the latent period. During this time, there is no obvious host response, and evidence of the presence of the infecting agent may not be measurable or discernible. Measurable early immune responses in the host may appear shortly before the first signs and symptoms of disease, marking the end of the latent period. Incubation periods for a microorganism vary by route of pathogen inoculation and the infecting dose. For example, brucellosis may be contracted through direct contact with blood or infected organic material, ingestion of raw, unpasteurized dairy products, or through airborne transmission in a laboratory or abattoir. The various modes of transmission for Brucella spp. results in an incubation period for brucellosis that is highly
variable, ranging from 5 days to several months. Incubation periods for other common microorganisms are as follows: 1-4 days for the rhinovirus (the common cold) or influenza virus,122 5-7 days for herpes simplex virus,123 7-14 days for polio virus,124 6-21 days for measles virus,122 10-21 days for varicella-zoster virus (chickenpox virus),125 20-50 days for hepatitis A virus126 and the rabies virus,127 and 80-100 days for hepatitis B virus.128
variable, ranging from 5 days to several months. Incubation periods for other common microorganisms are as follows: 1-4 days for the rhinovirus (the common cold) or influenza virus,122 5-7 days for herpes simplex virus,123 7-14 days for polio virus,124 6-21 days for measles virus,122 10-21 days for varicella-zoster virus (chickenpox virus),125 20-50 days for hepatitis A virus126 and the rabies virus,127 and 80-100 days for hepatitis B virus.128
The communicable period is the time in the natural history of an infection during which transmission may take place. Generally, microorganisms that multiply rapidly and produce local infections are associated with short incubation periods. For example, enterotoxin-producing S aureus undergoes such rapid multiplication in unrefrigerated food that symptoms of food poisoning may become manifest within 1-6 hours of ingestion of the contaminated meal.129 Microorganisms that cause disease that depend on hematogenous spread or multiplication in distant organs tend to have longer incubation periods. HIV antibodies are generally detectable 1-3 months after the initial exposure, whereas the HIV-infected person might remain asymptomatic for years.130
PREVENTION AND CONTROL
Measures for the prevention and control of communicable disease are directed at various links in the chain of infection. These include interventions to (1) eliminate or contain the reservoirs of infectious agents or curtail the persistence (endemicity) of a microorganism in a specific setting, (2) interrupt the transmission of infectious agents, or (3) protect the host against infection and disease. Effective prevention and control of communicable diseases calls for a detailed knowledge of the epidemiology of infectious diseases in variety of settings and environments.
Modifying Environmental Reservoirs
Interventions chosen to modify a reservoir depend on whether the reservoir is animate or inanimate. Quarantine, the restriction of movement of individuals who have been exposed to a potentially transmissible agent for the entire incubation period of the infection, is rarely used to control human disease in healthcare settings and has been replaced, largely, by active surveillance of exposed individuals in acute care hospitals and LTC facilities. Animate reservoirs (ie, carriers) include HCP who are colonized with potential pathogens in their nares or hands, relatives (or pets) who visit patients in the hospital, and patients known to be colonized or infected with a particular healthcare pathogen and are moved from one unit to another within a given institution or are transferred from one hospital to another. Since disease is often subclinical, it may be difficult to recognize and separate silent carriers from susceptible persons.
Treatment of humans to eradicate carriage of transmissible pathogens that are typically found in healthcare settings has had variable success. For example, treatment to eradicate VRE often yields mixed results,131,132,133 whereas there has been some success in the eradication of MRSA among hospital inpatients134,135,136 and in the community.137 There are no compelling data that show an association between eradication of Gram-negative carriage among patients or HCP and reduced rates of transmission. Thus, removal of an individual HCP, known to be a reservoir for a potentially transmissible pathogen, from a healthcare setting (eg, bone marrow unit or surgical ICU) with susceptible patients might be the only control or preventive option. However, removal of a colonized healthcare provider should generally only be undertaken if the provider has been linked by epidemiology and molecular diagnostics to the patient infections (eg, MRSA, carbapenem-resistant Enterobacteriaceae [CRE], C difficile). Human carriers of transmissible pathogens may be isolated from susceptible individuals, who are not colonized or infected, for the duration of their stay at the institution or for as long as they harbor the microorganism.138 Finally, ethical issues arise when the decision is made to expose asymptomatic carriers or colonized but well persons to medical therapy that might have serious side effects, or render them susceptible to adverse events, such as HAIs, disease, or undue morbidity.
In healthcare settings, reservoirs of a transmissible pathogen might be limited solely to the inanimate environment. Thus, appropriate control measures might include removing contaminated fruit, flowers, intravenous infusates, hand lotions, toys, white coats, stethoscopes, or other objects deemed to be potential reservoirs; appropriate handling of sewage and medical waste per published guidelines; ensuring that scrupulous aseptic techniques are maintained during invasive procedures or catheter insertion; or eliminating the agent from the environmental niche (eg, work surfaces in an ICU, medicine preparation areas, or moisture reservoirs in mechanical ventilators) by chemical or physical means. In some healthcare settings, microorganisms such as VRE or C difficile, may remain endemic or persistent despite identification and appropriate treatment or elimination of reservoirs. Persistence of resistant microorganisms may require periodic enhanced environmental disinfection of the concerned unit to curtail the endemicity of the pathogen (eg, use of a UV-C device for terminal disinfection).139 The importance of modifying environmental reservoirs for the prevention and control of infectious disease is sustained by the fact that much of the reduction in disease and death from infectious diseases in the industrialized world during the 20th century has been attributed to purification of potable water by filtration and chlorination; improvements in the cooking, processing, and inspection of food; and advancements in housing, nutrition, and sanitary disposal of human waste.140
Interrupting Transmission
Many of the features of interventions necessary to interrupt the transmission of infection are identical to those included in the interventions necessary for modifying inanimate environmental reservoirs discussed above. The most important addition to the measures to eliminate environmental reservoirs described above has been in interventions to improve the behavioral changes necessary to ensure appropriate hand hygiene between tasks in the preparation of food, caring for children, and caring for the sick. In the control of HAIs, the use of appropriate barriers, including the use of gloves, gowns, and eye protection, has been emphasized to prevent the transmission of bloodborne pathogens (eg, HIV and hepatitis B) between
patients and HCPs, as has the use of high-filtration masks for protection from respiratory transmission of influenza or tuberculosis.141,142,143 Although one of the key measures for the prevention and control of HAIs remains consistent use of hand hygiene before, between, and after patient contacts in healthcare settings, compliance or adherence to hand washing protocols among healthcare professions—a behavioral attribute—remains less than ideal. This is not surprising since as far back as 1996, Goldmann et al. found that National Guidelines are not well studied by physicians, and, if they are read, they rarely are incorporated into everyday practice.144 Compounding the problem is the growing body of evidence that hand hygiene is but one factor in the complex interplay of host, agent, and the environment that facilitates disease transmission. For a microorganism like VRE, transmission is enabled by one or more of the following factors: (1) the degree of hand hygiene among HCP; (2) the inherent properties of the microorganism that enable it to remain viable for days to weeks on dry, inert environmental surfaces, coats, or ties; (3) the proportion of patients in the unit of concern who are colonized with VRE; (4) the proportion of patients who are inherently susceptible to infection; (5) the selective pressure of vancomycin use in the unit; and (6) adherence to prevention efforts among HCP. Given the above, it follows that complete adherence to a strict hand hygiene policy alone will not necessarily preclude intrahospital transmission of VRE. One method commonly used to interrupt transmission of pathogens in healthcare settings is the isolation of patients known to be colonized or infected with a particular pathogen in a separate area, so as to reduce the probability of transmission of infection to other patients. These methods may include the use of private rooms, isolation precautions, dedicated patient equipment, and the allocation patients to specific HCP to avoid transmission of the pathogen by the HCPs themselves.
patients and HCPs, as has the use of high-filtration masks for protection from respiratory transmission of influenza or tuberculosis.141,142,143 Although one of the key measures for the prevention and control of HAIs remains consistent use of hand hygiene before, between, and after patient contacts in healthcare settings, compliance or adherence to hand washing protocols among healthcare professions—a behavioral attribute—remains less than ideal. This is not surprising since as far back as 1996, Goldmann et al. found that National Guidelines are not well studied by physicians, and, if they are read, they rarely are incorporated into everyday practice.144 Compounding the problem is the growing body of evidence that hand hygiene is but one factor in the complex interplay of host, agent, and the environment that facilitates disease transmission. For a microorganism like VRE, transmission is enabled by one or more of the following factors: (1) the degree of hand hygiene among HCP; (2) the inherent properties of the microorganism that enable it to remain viable for days to weeks on dry, inert environmental surfaces, coats, or ties; (3) the proportion of patients in the unit of concern who are colonized with VRE; (4) the proportion of patients who are inherently susceptible to infection; (5) the selective pressure of vancomycin use in the unit; and (6) adherence to prevention efforts among HCP. Given the above, it follows that complete adherence to a strict hand hygiene policy alone will not necessarily preclude intrahospital transmission of VRE. One method commonly used to interrupt transmission of pathogens in healthcare settings is the isolation of patients known to be colonized or infected with a particular pathogen in a separate area, so as to reduce the probability of transmission of infection to other patients. These methods may include the use of private rooms, isolation precautions, dedicated patient equipment, and the allocation patients to specific HCP to avoid transmission of the pathogen by the HCPs themselves.
Protecting the Host
The risk of acquisition and transmission of infectious diseases among patient populations in healthcare settings is better characterized if the patients’ immune status or immune response is known. Immunization is the most effective method of individual and community protection against epidemic diseases and can be active or passive. Through active immunization, smallpox, one of the major global communicable diseases, was eradicated.145,146 Although polio has been eliminated from large areas, including all of the Americas, and indigenous transmission of wild poliovirus types 1 and 3 infection has been interrupted in all but three countries worldwide (Afghanistan, Nigeria, and Pakistan), there were 33 reported cases in 2018.124,147,148,149,150,151,152,153 The occurrences of other childhood diseases have been substantially reduced, including diphtheria, pertussis, tetanus, measles, mumps, rubella, and infections of H influenzae type B.154,155,156 Despite the reduction in these vaccine preventable diseases, recent outbreaks of measles, mumps, and pertussis have been reported in unvaccinated and under vaccinated children and adults in several geographic areas including the United States. This raises concern about the potential resurgence of these diseases and the potential for outbreaks in HCP and healthcare settings.
Since one of the main goals of epidemiology is to identify subgroups in the patient population that are at high risk for infection and disease, a knowledge of the vaccination status of patients is essential for the prevention of infection or disease. Institutional immunization programs have been recommended as part of the occupational health services of healthcare facilities for some time, but compliance for all HCP has only recently come under mandate. Evaluation of patients for immunization during hospital admission is another program widely recommended but incompletely implemented. The residual endemic problems and periodic outbreaks of these vaccine-preventable diseases in both populations at large and in healthcare institutions have been multifactorial, including the failure of the delivery programs for the vaccines and vaccine hesitancy. Failures in vaccine delivery programs have been due to poor funding, poor prioritization of the programs, the lack of political infrastructure, and the lack of organization of the vaccine effort. Vaccine hesitancy stems from lack of trust in public health agencies, coincidental temporal relationships to adverse health outcomes, and unfamiliarity with preventable diseases. This has led to outbreaks of measles, pertussis, Haemophilus influenzae type B, and pneumococcal disease causing unnecessary use of public resources and morbidity.157,158
Passive immunization with hyperimmune or standard immunoglobulins is a valuable intervention in a small group of diseases, including certain genetic and acquired immunodeficiency diseases, primary antibody deficiency disorders, hypogammaglobulinemia in chronic lymphocytic leukemia, measles, hepatitis A, varicella-zoster virus exposure, hepatitis B, and HIV infections in children.159 Hyperimmune globulin preparations are obtained from blood plasma donor pools preselected for high antibody content against a specific antigen (eg, hepatitis B immune globulin, varicella-zoster immune globulin, cytomegalovirus immune globulin, and respiratory syncytial virus immune globulin). Although active searches have been carried out for other kinds of immunomodulating agents (eg, interferons and cytokines) and biologics that heighten host immune function and protect the host from infection or disease, there are no data that indicate such treatment modalities play any significant role in the prevention and control of HAIs.
Administering antimicrobials to ensure the presence of an anti-infective agent at the site of a potential infection is a more recent addition to the control programs protecting the host. The use of a single dose or short course of preoperative antimicrobials to reduce the probability of a surgical site infection with agents commonly seen following certain procedures has become a standard part of surgical practice.160 Profound cellular and humoral immunosuppression may ensue in patients following chemotherapy or radiotherapy of certain malignancies or may be a consequence of the primary disease process. Therapyrelated immunosuppression occurs during or following bone marrow transplantation or may be a sequela of therapeutic regimens used to prevent rejection of transplanted organs. The use of local and systemic anti-infectives in these patients has either prevented infection or mitigated the duration and severity of infection, leading to reduced morbidity and mortality, and improved outcomes.161,162,163,164
The use of preprocedural (eg, surgery or dental) antimicrobial prophylaxis in individuals with a history of rheumatic heart disease is also a standard recommendation to prevent bacterial endocarditis.165,166 Unfortunately, one of the side effects of repeated short courses of antimicrobials has been the appearance of significant antimicrobial resistance to these agents among pathogens associated with HAIs.167 This problem has been aggravated by overprescribing of antimicrobials for nonbacterial infections by some practitioners, over-the-counter sale of antimicrobials in many parts of the world, and the use of subtherapeutic doses of antimicrobials as growth promoters in animal husbandry in the United States and other countries.168,169,170,171
The use of preprocedural (eg, surgery or dental) antimicrobial prophylaxis in individuals with a history of rheumatic heart disease is also a standard recommendation to prevent bacterial endocarditis.165,166 Unfortunately, one of the side effects of repeated short courses of antimicrobials has been the appearance of significant antimicrobial resistance to these agents among pathogens associated with HAIs.167 This problem has been aggravated by overprescribing of antimicrobials for nonbacterial infections by some practitioners, over-the-counter sale of antimicrobials in many parts of the world, and the use of subtherapeutic doses of antimicrobials as growth promoters in animal husbandry in the United States and other countries.168,169,170,171
INFECTION PREVENTION AND ANTIMICROBIAL STEWARDSHIP
Infection prevention and antimicrobial stewardship programs (ASP) both share the common goal of enhancing patient safety and improving patient outcomes. Antimicrobial stewardship refers to interventions designed to improve antimicrobial prescribing practices to optimize clinical outcomes and minimize adverse events. ASPs focus on choosing the right drug, dose, duration, and route of administration.172 The improper use of antimicrobials promotes the development of MDR organisms, CDI, and antibiotic-associated adverse events. With the rate of antimicrobial resistance outpacing the development of new drugs, enhancing antimicrobial stewardship and infection prevention methods are of utmost importance. The impact of implementing ASPs along with infection control measures, especially hand hygiene, has been associated with a reduction in incidence rates of antibiotic-resistant bacteria and CDI in hospitalized patients.173
The CDC has called on all hospitals across the country to implement ASPs and identified seven elements associated with successful ASPs for hospitals and LTC facilities including leadership commitment, accountability, drug expertise, action, tracking, reporting, and education.174 The four elements identified for outpatient facilities are commitment, action for policy and practice, tracking and reporting, and education, and expertise.175 Recommendations from IDSA/Society for Healthcare Epidemiology of America (SHEA) currently advise that ASPs incorporate education, facility-specific clinical practice guidelines, limiting the use of antibiotics associated with a high risk of CDI, increasing appropriate use of oral antibiotics for initial therapy, and timely transition from intravenous to oral antibiotics.172,174
Although these guidelines suggest implementing ASPs led by infectious disease physicians and pharmacists, however, some hospitals do not have infectious disease physicians on staff. In such cases, many stewardship interventions are led by clinical pharmacists, intensivists, and hospitalists with expertise in infectious diseases. Engaging additional specialties in stewardship efforts has potential benefit that may help promote interventions such as prescribing the correct dose, duration, and indication to improve patient outcomes, reduce the overall burden of antimicrobial resistance, and reduce the cost of healthcare. However, cost-effectiveness studies of specific ASP interventions and methods are limited.
Recent recommendations to create more integrated stewardship models have been suggested.172 More comprehensive stewardship models involve antimicrobial stewardship, infection prevention, and diagnostic stewardship approaches. The use of rapid molecular diagnostics may enable tailor made diagnostics to improve antimicrobial prescribing. Diagnostic stewardship is defined as the promotion of evidence-based utilization of diagnostic tests with the goals of improving quality of care and safely reducing costs.176 Diagnostic stewardship has emerged as a way to obtain an accurate diagnosis, which is linked with fewer false-positive results, more appropriate antibiotic use, reduced risk of promoting antimicrobial resistance, fewer adverse effects, shorter hospital stays, and reduced cost of care by decreasing unnecessary admissions and length of stays.177,178 Healthcare facilities are now starting efforts to improve diagnostic stewardship by promoting proper collection and handling of specimens, engaging in education of appropriate indications for testing, and using the EHR to help guide clinical decision-making. An example of this has been demonstrated with C difficile testing. Using testing preauthorization followed by education reinforcement with computer decision support, reductions in hospitalonset CDI rates and oral vancomycin use were observed.179
HEALTHCARE-ASSOCIATED INFECTIONS AND INFECTIOUS DISEASES
Inherent in the measures for the prevention and control of HAIs is the ongoing education of HCP in infection control practices and procedures through guidelines published by the CDC,180,181 and the implementation of surveillance measures to detect changes in the incidence or prevalence rates of infections caused by microorganisms commonly associated with HAIs. The acute care hospital (inpatient, outpatient, and ICU) settings and LTC and home healthcare facilities provide special settings for the interaction of the agents of infection and patients and HCPs. The ongoing study of the basic epidemiologic features of agenthost interactions in these environments has led to recommendations for wide application of, and extensive testing of, surveillance, prevention, and control programs, which have proven highly successful. Descriptions of the special features of the investigations and interventions of these programs are described in the chapters that follow. Despite decreases in overall rates of HAIs involving the bloodstream, respiratory tract, surgical wounds, and urinary tract, rates of infections caused by antimicrobial-resistant pathogens have been increasing across the United States. The CDC estimates that at least 2 049 442 illnesses and 23 000 deaths have occurred because of antibiotic resistance.182 Thus, control of antimicrobial resistance remains inextricably linked to the control of transmission of healthcare-associated, antimicrobial-resistant pathogens, and the infections they cause. The CDC threat report noted that world leaders have described antibiotic-resistant microorganisms as “nightmare bacteria” that “pose a catastrophic threat” to people in every country in the world.182 The myriad of articles in the medical literature about antimicrobial resistance has in effect helped explain this failure since much of the data originated in facilities that had implemented untried control programs or had instituted ineffective programs.
The ongoing study of the basic epidemiologic features of agent-host interactions in these environments has led to evidence-based recommendations for HAI surveillance and prevention and control programs that have proved highly successful. For example, the CDC established evidencebased guidelines to control the spread of MRSA and VRE in acute care settings. The tenets of the guidelines are based on identification and containment of spread through (1) active surveillance cultures to identify the reservoir for spread, (2) routine hand hygiene, (3) barrier precautions for patients known or suspected to be colonized or infected with epidemiologically important antimicrobial-resistant pathogens, (4) implementation of an ASP, and (5) decolonization or suppression of colonized patients.138 However, numerous studies since that time have now called into question the benefit of contact precautions, although many of these studies have been observational and with confounding factors. It is unclear and extensively debated whether patients who are only colonized and not with active infection should be isolated to prevent the spread of multidrug-resistant organisms (MDROs) and if screening for these colonized patients is necessary.183,184 Additionally, contact isolation has been associated with noninfectious adverse events. These adverse events include a negative psychological state for the patient in isolation and less frequent and shorter time with patients from HCPs, which could impact patient safety.185
Despite all of the resources put into surveillance activities for HAIs in facilities throughout the nation, several obstacles remain that hinder progress in the control of these infections. These obstacles include substantial variation in surveillance activities from one medical center to another, in the collection, aggregation, and use of surveillance data, and lack of designated staff and healthcare epidemiologists to proactively aggregate, manage, and analyze surveillance data, and apply the results effectively. Failure of healthcare facilities to use effective control measures or inconsistent implementation of such measures (eg, surveillance cultures not being performed as recommended) lastly lack of commitment among healthcare providers and administrative personnel alike in appreciating the fact that the initial outlay of financial resources that is necessary to employ healthcare epidemiologists and infection preventionists and executing surveillance activities and preventive measures results in improved patient outcomes and substantial savings.
In conclusion, epidemiologic methods can enhance and strengthen evidence-based infection prevention and control through the design and conduct of studies to ascertain risk factors for infection and disease, establish the appropriateness of laboratory testing (eg, the clinical significance of positive blood cultures), or determine best outcome correlates. In addition, familiarity with infectious disease epidemiology enables characterization of community-or HAIs, the pathogens that cause these infections and their respective antimicrobial susceptibility profiles, and risk factors that cause (or are associated with) infection. Such data allow cost-effective patient care in hospitals with adequate resources and enable development of logical, evidence-based preventive policies that could be applied to hospitals without sophisticated epidemiologic or laboratory support. Finally, the integration of epidemiologic and microbiologic principles is necessary for the development of robust surveillance systems to track emerging infections and antimicrobial resistance, to conduct effective infection control activities and outbreak investigations, and to inform clinical and public health decision-making, research, and management practices.
REFERENCES
1. Kelsey JL, Thompson WD, Evans AS. Methods in Observational Epidemiology. New York, NY: Oxford University Press, University of Illinois at Urbana-Champaign; 1986.
2. Bryant GD, Norman GR. Expressions of probability: words and numbers. N Engl J Med. 1980;302(7):411.
3. Celentano D. Gordis Epidemiology. London, England: Elsevier; 2019.
4. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. Chichester, UK: John Wiley & Sons; 2011.
5. Garner JS, et al. CDC definitions for nosocomial infections, 1988. Am J Infect Control. 1988;16(3):128-140.
6. Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36(5):309-332.
7. Pien BC, et al. The clinical and prognostic importance of positive blood cultures in adults. Am J Med. 2010;123(9):819-828.
8. Weinstein MP, et al. The clinical significance of positive blood cultures in the 1990s: a prospective comprehensive evaluation of the microbiology, epidemiology, and outcome of bacteremia and fungemia in adults. Clin Infect Dis. 1997;24(4):584-602.
9. Lark RL, et al. Four year prospective evaluation of nosocomial bacteremia: epidemiology, microbiology, and patient outcome. Diagn Microbiol Infect Dis. 2000;38(3):131-140.
10. Rosner B. Fundamentals of Biostatistics. Boston, MA: Cengage Learning; 2010.
11. Institute of Medicine Committee on Quality of Health Care in America; Kohn LT, Corrigan JM, Donaldson MS, eds. To Err is Human: Building a Safer Health System. Washington, DC: National Academies Press (US); 2000.
12. Dawson-Saunders B, Trapp RG. Basic & Clinical Biostatistics. New York, NY: McGraw-Hill; 2005.
13. Greenberg RS. Medical Epidemiology. 2010. [Place of publication not identified]: Cram 101.
14. Selvin S. Statistical Tools for Epidemiologic Research. Oxford, New York: Oxford University Press; 2011.
15. Merrill RM. Statistical Methods in Epidemiologic Research. Burlington, MA: Jones & Bartlett Learning; 2016.
16. Grobbee DE, Hoes AW. Clinical Epidemiology: Principles, Methods, and Applications for Clinical Research. Burlington, MA: Jones & Bartlett Learning; 2015.
17. Wiens J, Shenoy ES. Machine learning for healthcare: on the verge of a major shift in healthcare epidemiology. Clin Infect Dis. 2018;66(1):149-153.
18. Roth JA, et al. Introduction to machine learning in digital healthcare epidemiology. Infect Control Hosp Epidemiol. 2018;39(12):1457-1462.
19. Scott IA, et al. Machine learning in clinical practice: prospects and pitfalls. Med J Aust. 2019;211:203-205.e1.
20. Diekmann O, Heesterbeek H, Britton T. Mathematical Tools for Understanding Infectious Disease Dynamics. Princeton, NJ: Princeton University Press; 2017.
21. Johnson Y, Kaneene J. Epidemiology: From Recognition to Results. Hoboken, NJ: Wiley Blackwell; 2018:1-30.
22. Archibald LK, Jarvis WR. Health care-associated infection outbreak investigations by the Centers for Disease Control and Prevention, 1946-2005. Am J Epidemiol. 2011;174(11 suppl):S47-S64.
23. Sivaraman K, Venkataraman N, Cole AM. Staphylococcus aureus nasal carriage and its contributing factors. Future Microbiol. 2009;4(8):999-1008.
24. Mercante JW, Winchell JM. Current and emerging Legionella diagnostics for laboratory and outbreak investigations. Clin Microbiol Rev. 2015;28(1):95-133.
25. Shaikh AA, et al. Evaluation of a low-intensity ultraviolet-C radiation device for decontamination of computer keyboards. Am J Infect Control. 2016;44(6):705-707.
26. Buffet-Bataillon S, et al. Outbreak of Serratia marcescens in a neonatal intensive care unit: contaminated unmedicated liquid soap and risk factors. J Hosp Infect. 2009;72(1):17-22.
27. Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins (LWW); 2011:1-758.
28. Crede W, Hierholzer WJ Jr. Surveillance for quality assessment: I. Surveillance in infection control success reviewed. Infect Control Hosp Epidemiol. 1989;10(10):470-474.
29. McGeer A, Crede W, Hierholzer WJ Jr. Surveillance for quality assessment: II. Surveillance for noninfectious processes: back to basics. Infect Control Hosp Epidemiol. 1990;11(1): 36-41.
30. Hoeprich PD, ed. Infectious Diseases. Hagerstown, MD: Harper & Row; 1972.
31. Crede WB, Hierholzer WJ Jr. Surveillance for quality assessment: III. The critical assessment of quality indicators. Infect Control Hosp Epidemiol. 1990;11(4):197-201.
32. Thomas C. Intrinsic and extrinsic sources and prevention of infection (in surgery). Surgery (Oxford). 2019;37(1):26-32.
33. Koutlakis-Barron I, Hayden TA. Essentials of infection prevention in the pediatric population. Int J Pediatr Adolesc Med. 2016;3(4):143-152.
34. Buettner P, Muller R. Epidemiology. Victoria, Australia: Oxford University Press; 2015.
35. Burnham JP, Feldman MF, Calix JJ. Seasonal changes in the prevalence of antibiotic-susceptible acinetobacter calcoaceticus-baumannii complex isolates result in increased multidrug resistance rates during winter months. Open Forum Infect Dis. 2019;6(6):ofz245.
36. MacVane SH. Antimicrobial resistance in the intensive care unit: a focus on gram-negative bacterial infections. J Intensive Care Med. 2017;32(1):25-37.
37. Sader HS, et al. Antimicrobial susceptibility of Gram-negative organisms isolated from patients hospitalized in intensive care units in United States and European hospitals (2009-2011). Diagn Microbiol Infect Dis. 2014;78(4):443-448.
38. Centers for Disease Control and Prevention (CDC). Staphylococcus aureus resistant to vancomycin—United States, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(26):565-567.
39. Centers for Disease Control and Prevention (CDC). Vancomycin-resistant Staphylococcus aureus—New York, 2004. MMWR Morb Mortal Wkly Rep. 2004;53(15):322-323.
40. Allegranzi B, Pittet D. Role of hand hygiene in healthcare-associated infection prevention. J Hosp Infect. 2009;73(4):305-315.
41. Nseir S, et al. Risk of acquiring multidrug-resistant Gram-negative bacilli from prior room occupants in the intensive care unit. Clin Microbiol Infect. 2011;17(8):1201-1208.
42. Teerawattanapong N, et al. Prevention and control of multidrug-resistant gram-negative bacteria in adult intensive care units: a systematic review and network meta-analysis. Clin Infect Dis. 2017;64(suppl_2):S51-S60.
43. Nicolle LE. Infection prevention issues in long-term care. Curr Opin Infect Dis. 2014;27(4):363-369.
44. Siroky MB. Pathogenesis of bacteriuria and infection in the spinal cord injured patient. Am J Med. 2002;113(suppl 1A): 67s-79s.
45. Bearman G, et al. Healthcare personnel attire in non-operatingroom settings. Infect Control Hosp Epidemiol. 2014;35(2): 107-121.
46. Bearman GM, et al. A controlled trial of universal gloving versus contact precautions for preventing the transmission of multidrug-resistant organisms. Am J Infect Control. 2007;35(10):650-655.
47. Kuruno N, Kasahara K, Mikasa K. Hand hygiene compliance in a universal gloving setting. Am J Infect Control. 2017;45(8): 830-834.
48. Brouqui P, et al. Infection control in the management of highly pathogenic infectious diseases: consensus of the European Network of Infectious Disease. Lancet Infect Dis. 2009;9(5): 301-311.
49. Hill AB. The environment and disease: association or causation? 1965. J R Soc Med. 2015;108(1):32-37.
50. Erichsen Andersson A, et al. Comparison between mixed and laminar airflow systems in operating rooms and the influence of human factors: experiences from a Swedish orthopedic center. Am J Infect Control. 2014;42(6):665-669.
51. Takayama Y, et al. A foodborne outbreak of a group A streptococcal infection in a Japanese university hospital. Eur J Clin Microbiol Infect Dis. 2009;28(3):305-308.
52. Siberry GK, et al. Guidelines for the prevention and treatment of opportunistic infections in HIV-exposed and HIV-infected children: recommendations from the National Institutes of Health, Centers for Disease Control and Prevention, the HIV Medicine Association of the Infectious Diseases Society of America, the Pediatric Infectious Diseases Society, and the American Academy of Pediatrics. Pediatr Infect Dis J. 2013;32(suppl 2):i-KK4.
53. Batchelor BI, et al. Biochemical mis-identification of Brucella melitensis and subsequent laboratory-acquired infections. J Hosp Infect. 1992;22(2):159-162.
54. Davey P, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev. 2017;2(2):CD003543.
55. Saha SK, Hawes L, Mazza D. Improving antibiotic prescribing by general practitioners: a protocol for a systematic review of interventions involving pharmacists. BMJ Open. 2018;8(4):e020583.
56. Bell BG, et al. A systematic review and meta-analysis of the effects of antibiotic consumption on antibiotic resistance. BMC Infect Dis 2014;14:13.
57. File TM Jr, Solomkin JS, Cosgrove SE. Strategies for improving antimicrobial use and the role of antimicrobial stewardship programs. Clin Infect Dis. 2011;53(suppl 1):S15-S22.
58. Pagani L, et al. Navigating the Web in search of resources on antimicrobial stewardship in health care institutions. Clin Infect Dis. 2009;48(5):626-632.
59. Pittet D, Landelle C, Harbarth SA. The Intensive Care Unit, Part A: HAI, Epidemiology, Risk Factors, Surveillance, Engineering and Administrative Infection Control Practices, and Impact. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2014.
60. Druge S, et al. Risk factors and the resistance mechanisms involved in Pseudomonas aeruginosa mutation in critically ill patients. J Intensive Care 2019;7:36.
61. McDermott H, et al. Vancomycin-Resistant Enterococci (VRE) in the intensive care unit in a nonoutbreak setting: identification of potential reservoirs and epidemiological associations between patient and environmental VRE. Infect Control Hosp Epidemiol. 2018;39(1):40-45.
62. Tice AD, et al. Practice guidelines for outpatient parenteral antimicrobial therapy. IDSA guidelines. Clin Infect Dis. 2004;38(12):1651-1672.
63. Berrevoets MAH, et al. Quality indicators for appropriate outpatient parenteral antimicrobial therapy (OPAT) in adults: a systematic review and RAND-modified Delphi procedure. Clin Infect Dis. 2020;70:1075-1082.
64. Saini E, et al. Early infectious disease outpatient follow-up of outpatient parenteral antimicrobial therapy patients reduces 30-day readmission. Clin Infect Dis. 2019;69:865-868.
65. Shannon KL, et al. Subclinical Plasmodium falciparum infections act as year-round reservoir for malaria in the hypoendemic Chittagong Hill districts of Bangladesh. Int J Infect Dis. 2016;49:161-169.
66. Di Domenico EG, et al. Biofilm producing salmonella typhi: chronic colonization and development of gallbladder cancer. Int J Mol Sci. 2017;18(9).
67. Scott L, et al. Diagnosis of opportunistic infections: HIV co-infections—tuberculosis. Curr Opin HIV AIDS. 2017;12(2):129-138.
68. Palmieri F, et al. Pulmonary tuberculosis in HIV-infected patients presenting with normal chest radiograph and negative sputum smear. Infection. 2002;30(2):68-74.
69. Wertheim HF, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5(12):751-762.
70. Kluytmans JA, Wertheim HF. Nasal carriage of Staphylococcus aureus and prevention of nosocomial infections. Infection. 2005;33(1):3-8.
71. Mehraj J, et al. Epidemiology of staphylococcus aureus nasal carriage patterns in the community. Curr Top Microbiol Immunol. 2016;398:55-87.
72. Liu Z, et al. Nasal decontamination for the prevention of surgical site infection in Staphylococcus aureus carriers. Cochrane Database Syst Rev. 2017;5:Cd012462.
73. Feng DY, et al. Differences in microbial etiology between hospital-acquired pneumonia and ventilator-associated pneumonia: a single-center retrospective study in Guang Zhou. Infect Drug Resist 2019;12:993-1000.
74. Barbier F. et al. Hospital-acquired pneumonia and ventilatorassociated pneumonia: recent advances in epidemiology and management. Curr Opin Pulm Med. 2013;19(3):216-228.
75. Jones RN. Microbial etiologies of hospital-acquired bacterial pneumonia and ventilator-associated bacterial pneumonia. Clin Infect Dis. 2010;51(suppl 1):S81-S87.
76. Kalil AC, et al. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis. 2016;63(5): e61-e111.
77. Mejia R, Nutman TB. Screening, prevention, and treatment for hyperinfection syndrome and disseminated infections caused by Strongyloides stercoralis. Curr Opin Infect Dis. 2012;25(4):458-463.
78. Nutman TB. Human infection with Strongyloides stercoralis and other related Strongyloides species. Parasitology. 2017;144(3):263-273.
79. Toledo R, Munoz-Antoli C, Esteban JG. Strongyloidiasis with emphasis on human infections and its different clinical forms. Adv Parasitol. 2015;88:165-241.
80. Evans RS, et al. Computerized identification of patients at high risk for hospital-acquired infection. Am J Infect Control. 1992;20(1):4-10.
81. Evans RS, et al. Computer surveillance of hospital-acquired infections: a 25 year update. AMIA Annu Symp Proc. 2009;2009: 178-182.
82. Burke JP, et al. The HELP system and its application to infection control. J Hosp Infect. 1991;18(suppl A):424-431.
83. McDonald LC, et al. Clinical practice guidelines for clostridium difficile infection in adults and children: 2017 update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018;66(7):e1-e48.
84. Bobulsky GS, et al. Clostridium difficile skin contamination in patients with C. difficile-associated disease. Clin Infect Dis. 2008;46(3):447-450.
85. Mayfield JL, et al. Environmental control to reduce transmission of Clostridium difficile. Clin Infect Dis. 2000;31(4):995-1000.
86. Yeager AS. Longitudinal, serological study of cytomegalovirus infections in nurses and in personnel without patient contact. J Clin Microbiol. 1975;2(5):448-452.
87. Ahlfors K, et al. Risk of cytomegalovirus infection in nurses and congenital infection in their offspring. Acta Paediatr Scand. 1981;70(6):819-823.
88. Bush C, et al. Bloodborne pathogen exposures: difference in reporting rates and individual predictors among health care personnel. Am J Infect Control. 2017;45(4):372-376.
89. U.S. Public Health Service. Updated U.S. public health service guidelines for the management of occupational exposures to HBV, HCV, and HIV and recommendations for postexposure prophylaxis. MMWR Recomm Rep. 2001;50(Rr-11):1-52.
90. Bennett NJ, et al. Occupational exposures to bloodborne pathogens in smaller hospitals. Infect Control Hosp Epidemiol. 2007;28(7):896-898.
91. Beekmann SE, Henderson DK. Protection of healthcare workers from bloodborne pathogens. Curr Opin Infect Dis. 2005;18(4):331-336.
92. Bennett SN, et al. Postoperative infections traced to contamination of an intravenous anesthetic, propofol. N Engl J Med. 1995;333(3):147-154.
93. Kainer MA, et al. Clostridium infections associated with musculoskeletal-tissue allografts. N Engl J Med. 2004;350 (25):2564-2571.
94. Collins SM, et al. Contamination of the clinical microbiology laboratory with vancomycin-resistant enterococci and multidrug-resistant Enterobacteriaceae: implications for hospital and laboratory workers. J Clin Microbiol. 2001;39(10):3772-3774.
95. Tablan OC, et al. Guidelines for preventing health-care—associated pneumonia, 2003: recommendations of CDC and the Healthcare Infection Control Practices Advisory Committee. MMWR Recomm Rep. 2004;53(Rr-3):1-36.
96. Moehring RW, et al. Outbreak of bacteremia due to Burkholderia contaminans linked to intravenous fentanyl from an institutional compounding pharmacy. JAMA Intern Med. 2014;174(4):606-612.
97. Lanini S, et al. Molecular epidemiology of a Pseudomonas aeruginosa hospital outbreak driven by a contaminated disinfectant-soap dispenser. PLoS One. 2011;6(2):e17064.
98. O’Neill E, Humphreys H. Surveillance of hospital water and primary prevention of nosocomial legionellosis: what is the evidence? J Hosp Infect. 2005;59(4):273-279.
99. Sydnor ERM, et al. Electronic-eye faucets: legionella species contamination in healthcare settings. Infect Control Hosp Epidemiol. 2012;33(3):235-240.
100. Kanamori H, Weber DJ, Rutala WA. Healthcare outbreaks associated with a water reservoir and infection prevention strategies. Clin Infect Dis. 2016;62(11):1423-1435.
101. Cohen R, et al. Water faucets as a source of Pseudomonas aeruginosa infection and colonization in neonatal and adult intensive care unit patients. Am J Infect Control. 2017;45(2):206-209.
102. Trautmann M, et al. Tap water colonization with Pseudomonas aeruginosa in a surgical intensive care unit (ICU) and relation to Pseudomonas infections of ICU patients. Infect Control Hosp Epidemiol. 2001;22(1):49-52.
103. Greene VW, et al. Microbiological contamination of hospital air. I. Quantitative studies. Appl Microbiol. 1962;10:561-566.
104. Greene VW, et al. Microbiological contamination of hospital air. II. Qualitative studies. Appl Microbiol. 1962;10:567-571.
105. Punjabi CD, Perloff SR, Zuckerman JM. Preventing transmission of mycobacterium tuberculosis in health care settings. Infect Dis Clin North Am. 2016;30(4):1013-1022.
106. Whiley H, Bentham R, Brown MH. Legionella persistence in manufactured water systems: pasteurization potentially selecting for thermal tolerance. Front Microbiol. 2017;8:1330.
107. Wooley DP, Byers KB. Biological Safety: Principles and Practices. Washington, DC: ASM Press; 2017.
108. Tellier R, et al. Recognition of aerosol transmission of infectious agents: a commentary. BMC Infect Dis. 2019;19(1):101.
109. Contaminated Heater-Cooler Devices. 2019 [cited 2019] https://www.cdc.gov/hai/outbreaks/heater-cooler.html
110. Sommerstein R, et al. Mycobacterium chimaera outbreak associated with heater-cooler devices: piecing the puzzle together. Infect Control Hosp Epidemiol. 2017;38(1):103-108.
111. Centers for Disease Control and Prevention (CDC). Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000-2004. MMWR Morb Mortal Wkly Rep. 2006;55(11):301-305.
112. Migliori GB, et al. Multidrug-resistant and extensively drugresistant tuberculosis in the West. Europe and United States: epidemiology, surveillance, and control. Clin Chest Med. 2009;30(4):637-665, vii.
113. Donald PR, van Helden PD. The global burden of tuberculosis—combating drug resistance in difficult times. N Engl J Med. 2009;360(23):2393-2395.
114. Kuijper EJ, Coignard B, Tull P. Emergence of Clostridium difficile-associated disease in North America and Europe. Clin Microbiol Infect. 2006;12(suppl 6):2-18.
115. Archibald LK, Banerjee SN, Jarvis WR. Secular trends in hospital-acquired Clostridium difficile disease in the United States, 1987-2001. J Infect Dis. 2004;189(9):1585-1589.
116. Iudicello S, Fadda A. A road map to a comprehensive regulation on ventilation technology for operating rooms. Infect Control Hosp Epidemiol. 2013;34(8):858-860.
117. Kainer MA, et al. Saline-filled breast implant contamination with curvularia species among women who underwent cosmetic breast augmentation. J Infect Dis. 2005;192(1):170-177.
118. Williamson DA, et al. Persistence, discordance and diversity of staphylococcus aureus nasal and oropharyngeal colonization in school-aged children. Pediatr Infect Dis J. 2016;35(7):744-748.
119. Mertz D, et al. Exclusive Staphylococcus aureus throat carriage: at-risk populations. Arch Intern Med. 2009;169(2): 172-178.
120. Schorderet-Weber S, et al. Blocking transmission of vectorborne diseases. Int J Parasitol Drugs Drug Resist. 2017;7(1): 90-109.
121. Laroche M, Raoult D, Parola P. Insects and the transmission of bacterial agents. Microbiol Spectr. 2018;6(5).
122. Lessler J, et al. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect Dis. 2009;9(5):291-300.
123. Chayavichitsilp P, et al. Herpes simplex. Pediatr Rev. 2009; 30(4):119-129; quiz 130.
124. Polio Elimination in the United States. 2017 [cited 2019]. https://www.cdc.gov/polio/us/hcp.html
125. Chickenpox (Varicella). 2018 [cited 2019]. https://www.cdc.gov/chickenpox/hcp/index.html
126. Neefe JR, Gellis SS, Stokes J Jr. Homologous serum hepatitis and infectious (epidemic) hepatitis; studies in volunteers bearing on immunological and other characteristics of the etiological agents. Am J Med. 1946;1:3-22.
127. The Path of the Rabies Virus. 2017 [cited 2019]. https://www.cdc.gov/rabies/transmission/body.html
128. Krugman S, et al. Viral hepatitis, type B. Studies on natural history and prevention re-examined. N Engl J Med. 1979;300(3): 101-106.
129. Argudin MA, Mendoza MC, Rodicio MR. Food poisoning and Staphylococcus aureus enterotoxins. Toxins (Basel). 2010; 2(7):1751-1773.
130. Rosenberg NE, et al. How can we better identify early HIV infections? Curr Opin HIV AIDS. 2015;10(1):61-68.
131. Sohn KM, Cheon S, Kim YS. Can fecal microbiota transplantation (FMT) eradicate fecal colonization with vancomycinresistant enterococci (VRE)? Infect Control Hosp Epidemiol. 2016;37(12):1519-1521.
132. Dinh A, et al. Clearance of carbapenem-resistant Enterobacteriaceae vs vancomycin-resistant enterococci carriage after faecal microbiota transplant: a prospective comparative study. J Hosp Infect. 2018;99(4):481-486.
133. Davido B, et al. Is faecal microbiota transplantation an option to eradicate highly drug-resistant enteric bacteria carriage? J Hosp Infect. 2017;95(4):433-437.
134. Vos MC, et al. 5 years of experience implementing a methicillin-resistant Staphylococcus aureus search and destroy policy at the largest university medical center in the Netherlands. Infect Control Hosp Epidemiol. 2009;30(10): 977-984.
135. Blok HE, et al. Role of healthcare workers in outbreaks of methicillin-resistant Staphylococcus aureus: a 10-year evaluation from a Dutch university hospital. Infect Control Hosp Epidemiol. 2003;24(9):679-685.
136. Henderson DK. Managing methicillin-resistant staphylococci: a paradigm for preventing nosocomial transmission of resistant organisms. Am J Infect Control. 2006;34(5 suppl 1):S46-S54; discussion S64-S73.
137. Henderson A, Nimmo GR. Control of healthcare- and community-associated MRSA: recent progress and persisting challenges. Br Med Bull. 2018;125(1):25-41.
138. Siegel JD, et al. 2007 guideline for isolation precautions: preventing transmission of infectious agents in health care settings. Am J Infect Control. 2007;35(10 suppl 2): S65-S164.
139. Disinfection and Sterilization. 2019 [cited 2019]. https://www.cdc.gov/infectioncontrol/guidelines/disinfection/
140. Rutala W, Weber DJ. Disinfection, sterilization, and control of hospital waste. In: Principles and Practice of Infectious Diseases. Philadelphia, PA: Churchill Livingston Elsevier; 2009:3677-3695.
141. Honda H, Iwata K. Personal protective equipment and improving compliance among healthcare workers in high-risk settings. Curr Opin Infect Dis. 2016;29(4):400-406.
142. Doll M, et al. Acceptability and necessity of training for optimal personal protective equipment use. Infect Control Hosp Epidemiol. 2017;38(2):226-229.
143. Boyce JM, Pittet D. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA hand hygiene task force. Infect Control Hosp Epidemiol. 2002;23(12 suppl):S3-S40.
144. Goldmann DA, Huskins WC. Control of nosocomial antimicrobial-resistant bacteria: a strategic priority for hospitals worldwide. Clin Infect Dis. 1997;24(suppl 1):S139-S145.
145. Dahlem Workshop on the Eradication of Infectious Diseases; Dowdle WR, Hopkins DR. The Eradication of Infectious Diseases: Report of the Dahlem Workshop on the Eradication of Infectious Diseases, Berlin, March 16-22, 1997. Chichester; New York: John Wiley & Sons.
146. Weiss RA, Esparza J. The prevention and eradication of smallpox: a commentary on Sloane (1755) ‘An account of inoculation’. Philos Trans R Soc Lond B Biol Sci. 2015;370(1666):20140378.
147. Lopalco PL. Wild and vaccine-derived poliovirus circulation, and implications for polio eradication. Epidemiol Infect. 2017;145(3):413-419.
148. Ghafoor S, Sheikh N. Eradication and current status of poliomyelitis in Pakistan: ground realities. J Immunol Res. 2016;2016:6837824.
149. Elhamidi Y, et al. Progress toward poliomyelitis eradication—Pakistan, January 2016-September 2017. MMWR Morb Mortal Wkly Rep. 2017;66(46):1276-1280.
150. Adamu US, et al. Progress toward poliomyelitis eradication—Nigeria, January 2018-May 2019. MMWR Morb Mortal Wkly Rep. 2019;68(29):642-646.
151. Martinez M, et al. Progress toward poliomyelitis eradication—Afghanistan, January 2017-May 2018. MMWR Morb Mortal Wkly Rep. 2018;67(30):833-837.
152. Our Progress Against Polio. 2017 November 3, 2017 [cited 2019]. https://www.cdc.gov/polio/progress/index.htm
153. World Health Organization. Poliomyelitis. 22 July, 2019 [cited 2019]. https://www.who.int/en/news-room/fact-sheets/detail/poliomyelitis
154. McLean HQ, et al. Prevention of measles, rubella, congenital rubella syndrome, and mumps, 2013: summary recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep. 2013;62(Rr-04):1-34.
155. Martinelli D, et al. Impact of Haemophilus influenzae type b conjugate vaccination on hospitalization for invasive disease in children fifteen years after its introduction in Italy. Vaccine. 2017;35(46):6297-6301.
156. Greenberg DP, et al. Epidemiology of pertussis and Haemophilus influenzae type b disease in Canada with exclusive use of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b pediatric combination vaccine and an adolescent-adult tetanus-diphtheria-acellular pertussis vaccine: implications for disease prevention in the United States. Pediatr Infect Dis J. 2009;28(6): 521-528.
157. Edwards KM, Hackell JM. Countering vaccine hesitancy. Pediatrics. 2016;138(3).
158. Salmon DA, et al. Vaccine hesitancy: causes, consequences, and a call to action. Am J Prev Med. 2015;49(6 suppl 4):S391-S398.
159. Young MK, Cripps AW. Passive immunization for the public health control of communicable diseases: current status in four high-income countries and where to next. Hum Vaccin Immunother. 2013;9(9):1885-1893.
160. Nishiwaki K, Ichikawa T. [WHO surgical safety checklist and guideline for safe surgery 2009]. Masui. 2014;63(3):246-254.
161. Gafter-Gvili A, et al. Antibiotic prophylaxis for bacterial infections in afebrile neutropenic patients following chemotherapy. Cochrane Database Syst Rev. 2012;1:CD004386.
162. Flowers CR, et al. Antimicrobial prophylaxis and outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(6):794-810.
163. Thursky KA, Worth LJ. Can mortality of cancer patients with fever and neutropenia be improved? Curr Opin Infect Dis. 2015;28(6):505-513.
164. Taplitz RA, Kennedy EB, Flowers CR. Outpatient management of fever and neutropenia in adults treated for malignancy: American Society of Clinical Oncology and Infectious Diseases Society of America Clinical Practice Guideline Update Summary. J Oncol Pract. 2018;14(4):250-255.
165. Wilson W, et al. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardio-vascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group. Circulation. 2007;116(15):1736-1754.
166. Cahill TJ, et al. Antibiotic prophylaxis for infective endocarditis: a systematic review and meta-analysis. Heart. 2017;103(12): 937-944.
167. Archibald L, et al. Antimicrobial resistance in isolates from inpatients and outpatients in the United States: increasing importance of the intensive care unit. Clin Infect Dis. 1997;24(2):211-215.
168. Hicks LA, et al. US outpatient antibiotic prescribing variation according to geography, patient population, and provider specialty in 2011. Clin Infect Dis. 2015;60(9): 1308-1316.
169. Centers for Disease Control and Prevention (CDC). Officerelated antibiotic prescribing for persons aged ≤14 years—United States, 1993-1994 to 2007-2008. MMWR Morb Mortal Wkly Rep. 2011;60(34):1153-1156.
170. Koji EM, Gebretekle GB, Tekle TA. Practice of over-the-counter dispensary of antibiotics for childhood illnesses in Addis Ababa, Ethiopia: a simulated patient encounter study. Antimicrob Resist Infect Control. 2019;8:119.
171. Bacanli M, Basaran N. Importance of antibiotic residues in animal food. Food Chem Toxicol. 2019;125:462-466.
172. Manning ML, et al. Antimicrobial stewardship and infection prevention-leveraging the synergy: a position paper update. Infect Control Hosp Epidemiol. 2018;39(4):467-472.
173. Baur D, et al. Effect of antibiotic stewardship on the incidence of infection and colonisation with antibiotic-resistant bacteria and Clostridium difficile infection: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(9):990-1001.
174. Pollack LA, Srinivasan A. Core elements of hospital antibiotic stewardship programs from the Centers for Disease Control and Prevention. Clin Infect Dis. 2014;59(suppl 3): S97-S100.
175. Sanchez GV, et al. Core elements of outpatient antibiotic stewardship. MMWR Recomm Rep. 2016;65(6):1-12.
176. Madden GR, Weinstein RA, Sifri CD. Diagnostic stewardship for healthcare-associated infections: opportunities and challenges to safely reduce test use. Infect Control Hosp Epidemiol. 2018;39(2):214-218.
177. Caliendo AM, et al. Better tests, better care: improved diagnostics for infectious diseases. Clin Infect Dis. 2013;57(suppl 3):S139-S170.
178. Messacar K, et al. Implementation of rapid molecular infectious disease diagnostics: the role of diagnostic and antimicrobial stewardship. J Clin Microbiol. 2017;55(3):715-723.
179. Christensen AB, et al. Diagnostic stewardship of C. difficile testing: a quasi-experimental antimicrobial stewardship study. Infect Control Hosp Epidemiol. 2019;40(3):269-275.
180. CDC Guidelines and Guidance Library. July 19, 2019 [cited 2019]. https://www.cdc.gov/infectioncontrol/guidelines/index.html
181. WHO Guidelines Approved by the Guidelines Review Committee. Guidelines on Core Components of Infection Prevention and Control Programmes at the National and Acute Health Care Facility Level. Geneva: World Health Organization; 2016. Copyright (c) World Health Organization 2016.
182. CDC. Antibiotic Resistance Threats in the United States, 2013. Atlanta, GA: Centers for Disease Control and Prevention, US Department of Health and Human Services. 2013.
183. Landelle C, Pagani L, Harbarth S. Is patient isolation the single most important measure to prevent the spread of multidrugresistant pathogens? Virulence. 2013;4(2):163-171.
184. Renaudin L, et al. Impact of discontinuing contact precautions for MRSA and ESBLE in an intensive care unit: a prospective noninferiority before and after study. Infect Control Hosp Epidemiol. 2017;38(11):1342-1350.
185. Abad C, Fearday A, Safdar N. Adverse effects of isolation in hospitalised patients: a systematic review. J Hosp Infect. 2010;76(2):97-102.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree