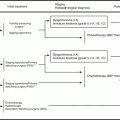
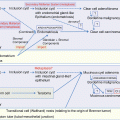
Fig. 12.1
The history of primary chemotherapy for epithelial ovarian cancer
In contrast to paclitaxel, fractionated weekly dosing of carboplatin remains a challenging regimen. The Multicentre Italian Trials in Ovarian Cancer (MITO) aimed to assess whether a weekly schedule of carboplatin plus paclitaxel was more effective than the same drugs given every 21 days (MITO-7) [23]. Patients with FIGO stage IC-IV ovarian cancer were randomly assigned to receive either carboplatin (AUC 6) plus paclitaxel (175 mg/m2) every 21 days for six cycles or carboplatin (AUC 2) or carboplatin (AUC 2) plus paclitaxel (60 mg/m2) every week for 18 weeks. Although the weekly schedule had lower toxicity and was better tolerated than the standard schedule, it did not appear to be associated with better PFS.
12.3 An Alternative Primary Chemotherapy
Docetaxel, a semisynthetic side-chain analogue of paclitaxel, was shown to be an active drug when delivered in the setting of platinum-resistant disease by a phase II trial [24]. One study by Scottish Randomized Trial in Ovarian Cancer (SCOTROC) directly compared carboplatin (AUC 5) plus paclitaxel (175 mg/m2) every 21 days with carboplatin (AUC 5) plus docetaxel (75 mg/m2). In this study, docetaxel-carboplatin was associated with a statistically higher incidence of grade 3–4 neutropenia, while the paclitaxel-carboplatin regimen resulted in a greater incidence of grade 3–4 peripheral neuropathy. However, the two regimens yielded similar efficacy in terms of PFS and response [25]. Thus, docetaxel (75 mg/m2) plus carboplatin (AUC 5) represents an alternative first-line chemotherapy regimen [12–14] (Fig. 12.1).
PLD plus carboplatin (PLD-C) is one of the options for patients who have difficulty taking taxane agents. According to the MITO-2 trial that evaluated whether PLD-C (carboplatin AUC 5, PLD 30 mg/m2) administered every 21 days was superior in terms of PFS to the standard TC regimen, there was no difference in response rate, PFS, or OS between the two regimens [26]. In view of the different toxicities (more hematologic adverse effects but less neurotoxicity and alopecia), PLD-C might be considered an alternative to standard therapy in some cases.
12.4 Adjuvant Chemotherapy for Early-Stage Ovarian Cancer
The use of adjuvant chemotherapy and its role in stage I ovarian cancer remains controversial. Several studies have been reported in which patients did not receive adjuvant chemotherapy after surgery. One prospective study enrolled 67 eligible patients with stage I ovarian cancer following accurate surgical staging. With a median follow-up time of 4 years, only one patient with clear cell carcinoma (CCC) experienced recurrence in the stage Ia and Ib group. The results of this study suggested that patients determined to have stage Ia or Ib cancer by accurate staging laparotomy and with histological grade 1 or 2 could be followed without adjuvant chemotherapy [27]. In another prospective randomized trial, 81 patients with histological grade 1 or 2 tumors and with stage Ia and Ib cancer on the basis of surgical resection plus comprehensive staging were assigned to receive either no chemotherapy or melphalan. As there were no significant differences between the two groups, this study concluded that adjuvant chemotherapy for patients with localized ovarian cancer who underwent comprehensive surgical staging could be omitted [28].
In 2003, two randomized controlled studies to evaluate the efficacy of adjuvant chemotherapy for early ovarian cancer were reported [29, 30]. In the ACTION1 trial, patients with ovarian cancer with stages Ia and Ib, grade II or III tumor; all grade of stages Ic-IIa; and all CCC, were randomly assigned to either observation or platinum-based chemotherapy following surgery [31]. This study showed that adjuvant chemotherapy statistically significantly improved the recurrence-free survival when all patients were taken into account; however, it was not effective in optimally staged patients. The ICON1 trial, in which patients with FIGO stage I disease constituted 93% of study subjects, also demonstrated that adjuvant chemotherapy with a platinum-based regimen improved survival and delayed recurrence [30]. The combined analysis of these two randomized clinical trials showed that OS at 5 years was 82% in the chemotherapy arm and 74% in the observation arm (HR = 0.67) and concluded that platinum-based adjuvant chemotherapy improved OS and recurrence-free survival at 5 years [31].
A meta-analysis that included five randomized controlled trials involving 1277 women with early-stage ovarian cancer indicated that women who received adjuvant platinum-based chemotherapy had better OS and PFS than those who did not (HR 0.71 and 0.65, respectively). In particular, women who were suboptimally staged or those who had high-risk disease received the greatest benefit from adjuvant chemotherapy. On the other hand, a benefit of adjuvant chemotherapy for women with adequate surgical staging and women with low- or intermediate-risk disease remained uncertain [32]. Taking these findings together, it has been generally accepted that adjuvant chemotherapy can be omitted in patients with stage IA or IB with grade 1 tumors.
12.5 Treatment Strategy by Histologic Subtype
It is currently unclear how to define treatment strategy by histologic subtype of ovarian cancer. TC have been recommended as primary chemotherapeutic agents for ovarian cancer based on the results of prior clinical studies [9, 10]. However, almost all patients enrolled in large randomized controlled trials of ovarian cancer have high-grade serous carcinoma, and the number of enrolled patients with CCC or mucinous carcinoma is limited. Several studies have demonstrated that CCC and mucinous carcinoma are less sensitive to platinum-based chemotherapy, and the median survival time of patients with these subtypes in advanced stages is significantly lower than patients with high-grade serous carcinoma [33, 34].
An in vitro study suggested that irinotecan (CPT-11) may be an effective agent for the treatment with CCC [35]. The JGOG and GCIG conducted the first randomized phase III trial of patients with CCC that compared irinotecan and cisplatin (CPT-P) with TC [36]. The JGOG3017/GCIG trial found that CPT-P provides no significant survival benefit to patients with CCC compared with TC. Treatment with existing anticancer agents has limitations for improving the prognosis of CCC. Therefore, another clinical trial using mTOR inhibitors (temsirolimus) is ongoing in patients with CCC, based on evidence that CCC expresses high levels of mTOR [37]. Mucinous carcinoma also demonstrates poor response to TC, and it has been suggested that mucinous carcinoma is more likely to be metastatic tumors from gastrointestinal cancer such as gastric and colon cancer than a primary ovarian tumor [38]. S-1 plus oxaliplatin has been assessed by clinical trial in patients with advanced gastric cancer. Thus, an oxaliplatin plus S-1 regimen is one of the treatment options for patients with mucinous carcinoma in ovary. Phase II clinical trials of oxaliplatin and S-1 for treating cases of advanced and recurrent mucinous ovarian cancer reported a 13% response rate and a 68% disease control rate (Shimada M, et al. Japan Society of Gynecologic and Obstetrics Annual Meeting 2013 abstract (unpublished date)).
Recently, The Cancer Genomic Atlas has revealed the genomic profiles of ovarian carcinoma [39], and the specific gene mutations in each histologic subtype are known [40]. Moreover, we can identify drug targets for those gene mutations by using the publicly available Genomics of Drug Sensitivity in Cancer drug database [41]. Over the past half century, ovarian cancer has been recognized as a single disease in clinical studies. However, the necessity of defining a treatment strategy by histological subtype has become a global consensus in ovarian cancer.
12.6 Neoadjuvant Chemotherapy
In patients diagnosed with ovarian cancer, the attempt is made to provide treatment with initial debulking surgery followed by chemotherapy. In cases in which the cancer has progressed so far that optimal surgery cannot be performed during the initial procedure, the goal is to minimize complications during the perisurgical stage and to improve complete excision rates as far as possible during this initial surgery. NAC may be considered to achieve these objectives.
Before 2010, most research regarding whether NAC contributes to improved prognosis following initial surgery was based on retrospective observational studies and involved differences in patient characteristics such as performance status (PS) and age. In a few non-randomized prospective studies, optimal surgery rates were found to improve [42, 43], perisurgical complications decreased [43–45] quality of life improved [46], and OS improved [42]. Two meta-analyses have compared prognosis based on whether surgery or NAC came first. One study showed poorer prognosis in the NAC group [47], while the other showed that although survival rates were equivalent, a greater percentage of patients received optimal surgery after NAC, and thus this treatment approach may be effective in improving prognosis [48].
The first prospective randomized study was reported in 2010 (EORTC 55971). In this study, 670 patients with stage IIIc to stage IV ovarian cancer, fallopian tube cancer, or peritoneal carcinoma were allocated to either a treatment group in which primary debulking surgery (PDS) was followed by at least six courses of the initial chemotherapy or another treatment group in which three courses of NAC preceded interval debulking surgery (IDS) that was then followed by a minimum of three additional courses of postsurgical chemotherapy. This was a noninferiority study in which the goal was to verify that the outcomes in the NAC group were not inferior to those in the PDS group; however, the median OS time was equal in both groups, with an OS of 29 months in the PDS group and 30 months in the NAC group [49]. When postoperative and perioperative complications and death rates were compared, the rates were significantly higher in the PDS group (severe hemorrhage 7.4% and deaths 2.5% vs. severe hemorrhage 4.1% and deaths 0.7% in the NAC group). This study concluded that NAC should be considered in cases in which complete resection is not possible with PDS alone. Furthermore, the authors stated that the most important prognostic factor in both groups is whether all macroscopic lesions can be completely resected during surgery.
The CHORUS study results, published in 2015, found NAC to be an acceptable form of standard therapy [50]. In this study, 552 patients believed to have stage III or stage IV ovarian cancer were allocated to either an NAC or a PDS group. Median survival times in the PDS and NAC groups were 22.6 months and 24.1 months, respectively, with an HR of 0.87, proving that NAC was noninferior. However, the median surgical time was very short in both groups at only 120 min, and the low optimal cytoreduction rates in the PDS group were mentioned as a factor.
Recently, the Japan Clinical Oncology Group published results comparing PDS to IDS in a phase III noninferiority trial (JCOG 0602). A total of 301 patients with Stage III and Stage IV ovarian cancer, fallopian tube cancer, or peritoneal carcinoma were allocated to either a PDS or an IDS group. The PDS group received eight courses of TC therapy after PDS, while IDS was performed after four courses of TC therapy, followed by four postsurgical courses of TC. In this study, surgical invasiveness was evaluated in addition to survival times. In the NAC group, there were fewer incidents requiring intestinal resection or resection of other organs due to complications. Moreover, a lower incidence of grade 3 or 4 postsurgical complications, smaller hemorrhage volumes, and albumin infusion requirements were noted in the NAC group. In this study, although surgical invasiveness was more limited in the NAC group, a similar survival time was achieved, suggesting that NAC may potentially become established as a standard form of therapy [51].
A growing body of evidence supports the use of NAC therapy in Stage IIIC and Stage IV ovarian cancer. In the future, it may be important to select an appropriate patient population in which NAC therapy is most likely to be effective. The objective of surgery in ovarian cancer is the complete resection of macroscopic lesions, and the clinician will need to choose between NAC and PDS from this perspective. Gastrointestinal resection and resection of other involved organs may become an issue in patient populations such as the elderly, those with low PS, or those in a poor nutritional state in which surgical invasiveness is an important consideration; NAC therapy is believed to be more appropriate in these patient groups [52–54] Evidence also suggests that patients with Stage IV cancer will benefit from NAC [55, 56].
12.7 Intraperitoneal Chemotherapy
As described above, current standard chemotherapy in ovarian cancer calls for IV administration of paclitaxel and carboplatin. However, most recurrent cases of ovarian cancer involve dissemination in the peritoneal cavity. Because direct IP administration allows more effective infiltration of cancer by antineoplastic drugs, researchers have investigated the efficacy of IP administration of cisplatin [57]. Since 1994, several randomized trials have been conducted on IP therapy [58–64] and significant improvements in survival rates were reported in three of those studies. In a review of reports in which taxanes were combined with platinum formulations (the current standard therapy), it was revealed that patients in the GOG114/SWOG9227 study comprised 462 cases with Stage III disease in which the residual tumor load after the initial surgery was ≤1 cm in diameter [63]. Patients were allocated into either an IV group treated with six courses of 135 mg/m2 of IV paclitaxel +75 mg/m2 of IV cisplatin or an IP group that received two courses of IV carboplatin (AUC 9) followed by six courses of 135 mg/m2 IV paclitaxel +100 mg/m2 of cisplatin IP. A significant increase in PFS was noted in the IP group compared to the IV group (28 months vs. 22 months, respectively), with increased OS of 63 months vs. 52 months. However, patients in the IP group received a higher dose of carboplatin for a greater number of courses, which was believed to be responsible for the increase in PFS, and since the IP group experienced a higher rate of toxicity, this form of administration could not be recommended as standard therapy. The GOG172 study compared 415 patients with Stage III cancer allocated into either an IV group treated with six courses of IV paclitaxel 135 mg/m2 + IV cisplatin 75 mg/m2 or an IP group administered 135 mg/m2 IV paclitaxel (Day 1) + 75 mg/m2 IP cisplatin (Day 2) + 60 mg/m2 IP paclitaxel (Day 8) [64]. In the IP group, significant increases were noted in both PFS (19 months vs. 24 months) and OS (49 months vs. 67 months); however, the cisplatin dose was higher in the IP group and an additional dose of paclitaxel was administered on Day 8, so it may be difficult to compare the superiority or inferiority of these treatments directly.
Based on these comparative studies, the US National Cancer Institute (NCI) and GOG conducted a meta-analysis and found that the risk of death decreased 21.5% with IP therapy vs. IV therapy. They announced in January 2006 that patients with ovarian cancer with optimally debulked FIGO Stage III ovarian cancer should receive counseling regarding the clinical benefit associated with combined IV and IP administration of chemotherapy [65, 66]. Long-term follow-up results from the GOG172 and GOG114 studies were reported in 2015 [67]. In these studies, the median duration of follow-up observation was 10.7 years, and the median survival time in the IV group was 51.4 months, whereas the median survival time in the IP group was 61.8 months. This indicates that there was a 23% reduction in risk of death in the IP group. This report strongly supports the efficacy of IP treatment.
However, it was pointed out that IP therapy involves higher toxicity and a lower completion rate. Furthermore, comparative studies to date are not direct comparisons of the administration methods, and a major issue is that carboplatin, the current standard treatment, was not used in the above studies.
A larger, Phase III clinical study on IP administration is currently underway to resolve these issues in study design. One phase II/III study known as the JGOG3019 (iPocc trial) involves PDS patients with Stage II to Stage IV disease. This study compares a dose-dense TC therapy group (paclitaxel 80 mg/m2 [Days 1, 8, and 15] + carboplatin AUC 6 [Day 1]) against a group receiving paclitaxel 80 mg/m2 IP or IV + carboplatin AUC 6 IP administration and should allow direct assessment of the efficacy of IP administration [68]. The GOG conducted the GOG252 study, in which concurrent bevacizumab and maintenance therapy were added to all study arms. GOG252 involved women with Stage II-III epithelial ovarian, fallopian tube, or primary peritoneal carcinoma. All patients underwent optimal surgical debulking to 1 cm or less residual disease. In this study, three arms were compared: dose-dense TC chemotherapy as the standard treatment, IP carboplatin alone, and the experimental arm in the GOG172 study as the investigational treatment group. Furthermore, the National Cancer Institute of Canada (NCIC) conducted a phase II/III clinical study in patients who underwent optimal surgery after NAC [69]. A three-arm study was designed as a Phase II clinical study in which arm one received IV paclitaxel 135 mg/m2 (Day 1) + IV carboplatin AUC 5 to 6 (Day 2) + IV paclitaxel 60 mg/m2 (Day 8). Arm two consisted of the experimental arm of the GOG172 study, while arm three received 135 mg/m2 IV paclitaxel (Day 1) + IP carboplatin AUC 5–6 (Day 2) + paclitaxel 60 mg/m2 IP (Day 8). The most superior form of IP therapy in the Phase II study was then compared to arm one in a Phase III clinical study. This study compared IP administration of cisplatin against carboplatin to determine which was more effective and also allowed assessment of whether IP therapy was more effective than IV administration.
Although it has been reported that the effect of IP therapy is superior to that of conventional TC therapy, there are also complications specific to this treatment, such as the risk of infection from catheter use, catheter occlusion, adhesions, and peritoneal irritation due to local exposure to antineoplastic agents. If the results of the Phase III studies described above become available, the risks and benefits of IP therapy may become clearer, allowing clinicians to establish appropriate indications and administration methods. IP therapy would then become an effective treatment method for advanced ovarian carcinoma.
12.8 Targeted Molecular Therapy
In recent years, molecularly targeted drugs have become widely used in cancer treatment and have played major roles in the treatment of ovarian cancer. The molecularly targeted drugs that have been reported to be effective in chemotherapy for ovarian cancer can be broadly classified into the following two groups: anti-angiogenic target agents and poly (ADP-ribose) polymerase (PARP) inhibitors. Bevacizumab is a widely used anti-vascular endothelial growth factor (VEGF) human monoclonal antibody that belongs in the former category. Olaparib is the most common example of the latter and is closely dependent on the presence of BRCA mutations. In this section, we will discuss molecularly targeted therapy used in initial chemotherapy and subsequent maintenance therapy.
12.8.1 Anti-Angiogenetic Target Therapies
Neovascularization plays a vital role in the progression of dissemination and metastases, as well as the production of ascites in ovarian cancer. VEGF and vascular endothelial growth factor receptor (VEGFR) are strongly expressed in ovarian cancer, and it has been reported that VEGF expression correlates with ovarian cancer progression and the development of ascites [70]. The most widely used molecularly targeted therapy worldwide against ovarian cancer is a monoclonal antibody against VEGF called bevacizumab (BEV). BEV is a drug that has been shown to be effective against other cancers such as colon cancer, lung cancer, and breast cancer, in addition to ovarian cancer.
In a clinical study conducted by GOG using BEV as the initial therapy (GOG218), patients with FIGO stage III to stage IV ovarian cancer were divided into three treatment arms. With this study design, arm one comprised six courses of TC therapy (administered every 3 weeks), arm two included six courses of TC therapy with BEV 15 mg per kilogram administered concurrently from the second course, and arm three was composed of six courses of TC therapy with BEV administered from the second course until the 15th month. The study results showed a significant increase in PFS (3.8 months) in arm three compared to the control arm; however, there were no differences in OS [71]. In addition, ICON 7 was a randomized study conducted in patients with Stage I to stage IV ovarian cancer in which TC therapy was compared to combined treatment with BEV + maintenance treatment. In this study, concurrent BEV was administered from the second course of TC chemotherapy at a dose of 7.5 mg/kg and continued for 12 courses after conclusion of the TC regimen. Compared to the control group, the TC + BEV group showed a significant increase in PFS (1.7 months) [72]. In addition, in cases of platinum-resistant recurrent ovarian cancer, a synergistic effect was confirmed with the addition of BEV [73]. Based on the above findings, added effects of BEV were confirmed both during initial therapy and recurrent therapy for ovarian cancer; however, both outcomes were merely increases in PFS, and thus far, no increases in OS have been observed.
Characteristic serious adverse events associated with BEV include gastrointestinal perforation, thromboembolism, hypertension, delayed wound healing, hemorrhage, proteinuria, and fistula. There was a high incidence of gastrointestinal perforation (11%; 5/44 cases) during the Phase II study in patients with ovarian cancer, resulting in premature discontinuation of the study. However, a retrospective analysis revealed that the significant risk factor for gastrointestinal perforation was the treatment history rather than any of the three treatment regimens [74]. In the GOG 218 study, patients with intestinal obstruction or a history of radiation therapy to the abdomen or pelvis were excluded from the study, and the incidence of gastrointestinal bleeding and perforation was 3.4% in the BEV administration group, which was higher than the 1.7% in the placebo group [71]. While BEV can be an effective drug that inhibits the production of ascites and alleviates symptoms that could markedly decrease the quality of life of patients with ovarian cancer, it can also cause serious adverse events such as gastrointestinal perforation. During the administration of BEV, more consideration should be given to the selection criteria and exclusion criteria in clinical trials to carefully select patients and monitor for adverse events.
Nintedanib is a tyrosine kinase inhibitor (TKI) that inhibits VEGFR, fibroblast growth factor receptor (FGFR), and platelet-derived growth factor receptor (PDGFR). In a Phase III clinical study, nintedanib was reported to have an additive effect on TC therapy in patients with Stage IIb to Stage IV ovarian cancer [75]. Patients in the nintedanib administration group ingested 200 mg of nintedanib for a maximum of 120 weeks. Compared to the placebo group, patients in the nintedanib group showed a significant increase in PFS (17.2 months vs. 16.6 months, HR = 0.84).
Pazopanib is a TKI that inhibits VEGFR, PDGFR, c-kit, and c-fms. Patients with Stage II–Stage IV ovarian cancer were investigated in an RCT to examine the effectiveness of maintenance therapy with pazopanib. When 800 mg of pazopanib was ingested for a maximum of 2 years, PFS was found to increase significantly compared to the placebo group (17.9 months vs. 12.3 months, HR = 0.77) [76]. However, the incidence of discontinuation of treatment on the basis of serious adverse events such as hypertension, neutropenia, liver dysfunction, and diarrhea was much higher in the pazopanib treatment group at 33.3% compared to the 5.6% observed in the control group. Regarding maintenance therapy with pazopanib, it has been reported to adversely affect OS in East Asian patients compared to non-East Asian patients [77].
12.8.2 Poly-Ribose Polymerase Inhibitor
The other effective molecular target is believed to be PARP. Genes can be damaged by a variety of factors, and PARP is a DNA-binding protein that detects and binds to sites of DNA damage (single-strand breaks, SSB), activating the base excision repair pathway. In humans, the PARP subfamily has 17 types; however, most of the DNA repair activity is handled by PARP-1, which has been widely studied [78]. PARP inhibitors express antitumor activity by inhibiting SSB DNA repair, so when these drugs were first developed, they were believed to potentiate the effects of chemotherapy. Subsequently, it was discovered that cell lines with BRCA 1/2 gene deletions were found to be 100- to 1000-fold more sensitive to PARP inhibitors compared to cell lines without BRCA mutations [79, 80]. PARP inhibitors were known to induce cell death in a manner known as synthetic lethality in cells with BRCA 1/2 gene dysfunction, drawing attention to PARP inhibitors as potential drugs with activity against ovarian cancer.
Olaparib was the first PARP inhibitor introduced into clinical use. The results of a randomized double-blind Phase II study were reported in patients with platinum-sensitive recurrent ovarian cancer or fallopian tube cancer or primary peritoneal carcinoma [81]. This study evaluated the effectiveness of maintenance therapy with olaparib. In a group that received 400 mg of olaparib for 8 weeks, PFS increased significantly in the olaparib group compared to the placebo group (8.4 months vs. 4.8 months, HR = 0.35). Furthermore, when patients were divided into a group with BRCA genetic abnormalities vs. those without genetic abnormalities to compare the efficacy of olaparib, a marked increase in PFS was noted in the group with BRCA genetic mutations (11.2 months vs. 4.3 months, HR = 0.18) [82]. Updated results were reported in 2016, revealing a statistically significant difference in PFS in the olaparib group compared to placebo (HR 0–35). Maximum effects were confirmed in the group with BRCA gene mutations (HR = 0.18) [83]. Based on the above findings, olaparib is an effective treatment for patients with advanced ovarian cancer with BRCA gene mutations and has been approved by the Food and Drug Administration (FDA), drawing attention to its use as a new therapeutic agent.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree