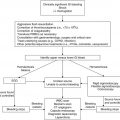
Recommendation
Grade
Comments
Reference
Data from studies of non-oncology patientsa
Data from studies of adult oncology patientsa
Data from studies of pediatric oncology patientsa
Antibacterial prophylaxis with a fluoroquinolone should be considered for pediatric patients with expected durations of prolonged and profound neutropenia
Not applicable
1A
0
Recommended for high-risk adult oncology patients; insufficient data in children to formulate a recommendation
Gafter-Gvili et al. (2012)
Antibiotic or ethanol locks should be considered for prevention of central line-related bacteremia
1B
1B
0
Specifics of antibiotic and ethanol lock therapies have varied across studies; insufficient data in children with cancer to formulate a recommendation
Chlorhexidine bathing should be considered for the prevention of central line-related bacteremia
1B
0
0
Insufficient data in oncology patients to formulate a recommendation
14.2 Prevention of Bacterial Infection
Bacterial infection is the leading cause of treatment-related morbidity and mortality in pediatric oncology patients. Evaluation and treatment of suspected or proven bacterial infection is a core component of care in children receiving myelosuppressive chemotherapy and is discussed extensively in Chap. 1. Strategies to prevent such infection, including both pharmacologic and non-pharmacologic approaches, are not as well established; data to support prophylactic measures, as well as gaps in our current knowledge, are the focus of this section.
14.2.1 Risk Stratification
As discussed in Chap. 1, the child with cancer may have multiple risk factors for serious bacterial infection including a central venous catheter, interruption of normal mucosal surfaces secondary to mucositis, surgical wounds and altered anatomy from tumor masses. Certain cancer predisposition syndromes may contribute to increased risk of infection. For example, children with Down syndrome and acute lymphoblastic leukemia (ALL) have a higher rate of infectious complications than those with ALL alone (Rabin et al. 2012). The factor most strongly associated with risk of serious bacterial infection is chemotherapy-related neutropenia; patients receiving the most intensive myelosuppressive regimens are at the highest risk. For example, 39–50 % of children treated on Children’s Cancer Group protocol 2961 for acute myelogenous leukemia (AML) had Gram-positive infections and 18–28 % had Gram-negative sterile site infections during the three phases of therapy (Sung et al. 2007). Treatment-related mortality from bacterial infection in children with AML is consistently 3–4 % across cooperative group studies over the last several decades (Woods et al. 1996; Riley et al. 1999; Creutzig et al. 2004; Sung et al. 2007, 2009). Similarly, children with relapsed ALL receiving intensive chemotherapy have high rates of infectious morbidity and mortality related to bacterial infection (Abshire et al. 2000; Lawson et al. 2000; Thomson et al. 2004; Raetz et al. 2008; Locatelli et al. 2009).
14.2.2 Antimicrobial Approaches
14.2.2.1 Adult Data
Antibacterial prophylaxis for afebrile patients receiving myelosuppressive chemotherapy is widely adopted in adult oncology practice based on data from trials preformed over the last 30 years with more than 100 published studies evaluating various antibiotic regimens. Two large contemporary studies in adult solid tumor, lymphoma and leukemia patients compared levofloxacin to placebo using a double-blind, randomized, controlled design (Bucaneve et al. 2005, Cullen et al. 2005). Although neither study was able to show a significantly decreased rate of death in the levofloxacin arm, both studies showed that prophylaxis significantly decreased episodes of fever, clinically documented infection and hospitalization. In solid tumor and lymphoma patients, Cullen et al. (2005) reported a 34.2 % rate of infection with levofloxacin compared to 41.5 % in the placebo arm (RR 0.82, 95 % CI 0.73–0.94, p = 0.004) while Bucaneve et al. (2005) reported 22 % infection rate in the levofloxacin arm versus 39 % with placebo (absolute risk difference –0.17, 95 % CI –0.24 to –0.10) in patients with leukemia, lymphoma and solid tumors.
Meta-analysis of randomized controlled trials has shown that prophylaxis has an impact on incidence of infection and, more important, is associated with a decreased risk of death (Gafter-Gvili et al. 2012). All-cause mortality was reduced in patients receiving prophylaxis (RR 0.66, 95 % CI 0.55–0.79), with the number of patients needed to treat to prevent death from any cause being 34 (95 % CI 26–56). The most substantial effect was found in studies that used fluoroquinolones (FQs) as prophylaxis with the greatest benefit in those at the highest risk.
14.2.2.2 Pediatric Data
Data regarding the utility of bacterial prophylaxis in neutropenic children are very limited. Early studies using trimethoprim-sulfamethoxazole, erythromycin, and amoxicillin-clavulanate were hampered by difficulties with patient accrual and compliance with oral therapies (Pizzo et al. 1983; van Eys et al. 1987; Castagnola et al. 2003). Studies which have documented benefit are limited by small patient numbers at single institutions. For instance, a single arm pilot trial of ciprofloxacin prophylaxis in children receiving a reinduction block of therapy for ALL showed a decreased incidence of hospitalization, bacteremia and intensive care unit admissions compared to historical controls (Yousef et al. 2004). Specifically, hospitalization was 90 % in the controls versus 58 % in the study group (p < 0.001), the overall rate of proven bacteremia was 22 % in the controls versus 9 % in the ciprofloxacin group (p = 0.028), and there were no Gram-negative bacteremias in this group as compared to 7.7 % in the controls (p < 0.001) (Yousef et al. 2004).
Similarly, a retrospective study in pediatric AML patients of prophylactic cefepime, or prophylactic vancomycin with ciprofloxacin or a cephalosporin, showed decreased rates of septicemia and hospital days compared to historical controls while patients who received only an oral cephalosporin as prophylaxis had no significant decrement in bacterial sepsis or hospital days compared with controls (Kurt et al. 2008). A recent survey by the Children’s Oncology Group (COG) of institutional standards for supportive care during AML trial AAML0531 found that antibacterial prophylaxis significantly reduced Gram-positive sterile site infection (incidence rate ratio [IRR] 0.71, 95 % CI 0.57–0.90, p = 0.004) with a trend toward reducing all bacterial infection (IRR 0.85, 95 % CI 0.72–1.01, p = 0.058) (Sung et al. 2013).
14.2.3 Risks of Prophylaxis
The main concern when considering prophylactic antibiotics is the potential development of bacterial resistance. Any exposure to antibiotics increases the risk of colonization and possible subsequent infection with resistant pathogens. Resistance can be acquired by selection of previously undetectable but present bacteria or de novo in a previously susceptible organism. Resistant pathogens can be transmitted from patient to patient. Studies performed in the 1980s in patients with leukemia and in those undergoing hematopoietic stem cell transplant (HSCT) documented that surveillance stool cultures could often detect pathogens preceding the development of bacteremia (Schimpff et al. 1972; Tancrede and Andremont 1985; Wingard et al. 1986). Patients identified as having a resistant organism in their stool were much more likely to have a subsequent infection with a resistant pathogen. Systematic studies evaluating the impact of FQ prophylaxis on the bacterial resistance profiles from sterile site cultures in oncology patients are limited. As anticipated with any broad antibacterial use, centers with extensive use of FQs have documented increased rates of clinically relevant FQ-resistant pathogens (Razonable et al. 2002; Kern et al. 2005; Prabhu et al. 2005). The two large contemporary double-blind studies of levofloxacin prophylaxis, which combined included 2,325 patients, did not note increased rates of resistant organisms from sterile site cultures; however, the studies were not powered to detect this outcome (Bucaneve et al. 2005; Cullen et al. 2005). Specifically, Bucaneve et al. (2005) noted 3 % of patients in the levofloxacin group had FQ-resistant Gram-negative bacilli as compared to 1 % in the placebo group (absolute risk difference 2 %, 95 % CI –0.4 % to 3 %, p = 0.10) while Cullen et al. (2005) did not routinely test for FQ sensitivity.
The use of antibacterial prophylaxis also has potential risk for other infectious complications. Exposure to FQs in adult oncology patients has been associated with increased incidence of Clostridium difficile-associated diarrhea (CDAD) (von Baum et al. 2006). Rates of CDAD in hospitalized pediatric patients remain significantly lower than their adult counterparts although has increased in the last decade (Kim et al. 2008a). Additionally, there is theoretical concern that antibacterial prophylaxis may increase the rate of invasive fungal infection (IFI) though the data available do not support this concern (Gafter-Gvili et al. 2012).
FQs are the class of antibiotic most intensively investigated for antibacterial prophylaxis in adult oncology patients; in meta-analysis, FQs are the agents associated with the greatest benefit (Gafter-Gvili et al. 2012). As a class their safety profile is similar to other antibiotics with unique toxicities including rare but consistent association with tendonitis and tendon rupture (with those >60 years of age and receiving concomitant steroids at greatest risk), as well as possible association with retinal detachment (Owens and Ambrose 2005; Etminan et al. 2012). Concern regarding potential FQ toxicity in children arose from early preclinical data that associated FQ exposure to articular cartilage damage in young animals although there is now significant literature describing the safety in children (Hampel et al. 1997; Jick 1997; Redmond 1997; Bradley et al. 2007; Schaad 2007, Noel et al. 2008). Toxicity analysis in more than 2,500 pediatric patients reported that levofloxacin exposure was associated with an increased rate (3.4 % vs. 1.8 %, p = 0.025) of musculoskeletal complaints (primarily arthralgia) at 12 months postexposure though the quality of symptoms was not different in the exposed and unexposed groups (Noel et al. 2007). Some concern remains that the results were biased by the open-label study design. Currently only ciprofloxacin is approved in the United States for limited indications in those <18 years of age by the Food and Drug Administration (FDA).
14.2.4 Guidelines and Current Usage of Antibacterial Prophylaxis
The Infectious Diseases Society of America (IDSA) guidelines recommend the use of FQ prophylaxis in high-risk adult patients, with high-risk being defined as anticipated prolonged and profound neutropenia (i.e., absolute neutrophil count [ANC] ≤ 0.1 × 109/L for >7 days) (Freifeld et al. 2011). Similarly, the National Comprehensive Cancer Network (NCCN) guidelines recommend FQ prophylaxis in patients whose infection risk is considered to be intermediate- or high-risk (i.e., neutropenia >7 days) (NCCN 2008). The paucity of data in children have precluded the generation of pediatric-specific recommendations and these guidelines do not address the use of prophylaxis in pediatric patients. Thus, survey data for pediatric AML patients show that only approximately 13 % receive routine antibacterial prophylaxis in North American settings (Lehrnbecher et al. 2009; Sung et al. 2013).
14.2.5 Central Venous Catheter-Related Interventions
Central venous catheters (CVCs) are a common site of infection in pediatric oncology patients and prophylactic methods including CVC care plans, lock therapy as well as chlorhexidine cleansing are reviewed briefly here. See Chap. 17 for a more detail review of CVC care.
14.2.5.1 Protocols for Line Placement and Care
Revised guidelines for the prevention of infection with intravascular catheters have been recently published (O’Grady et al. 2011). The strategies recommended include systems for training those involved in the placement and care of catheters, the use of maximal sterile barrier precautions at the time of line placement and using a >0.5 % chlorhexidine skin solution with alcohol for local antisepsis. The guidelines emphasize the quality assurance and safety aspects of standardized “bundles” of central line care and systems for evaluation of compliance with institutional standards.
14.2.5.2 Antibiotic and Ethanol Locks
Antibiotic and ethanol lock therapy involves using an antimicrobial agent to fill the lumen of the central venous catheter in an attempt to prevent line-related bacterial infections. A number of studies have shown efficacy for various antimicrobial agents used as lock therapy, including studies in children with cancer (Henrickson et al. 2000). A meta-analysis of randomized controlled studies comparing vancomycin/heparin lock versus heparin alone, which included five studies in children with cancer, showed a benefit to the use of antibiotic lock in prevention of infection (Safdar and Maki 2006). As with any antibacterial prophylactic strategy, use of the agent raises concerns for emergence of resistance which has been documented in a study of gentamicin central catheter locks (Landry et al. 2010).
Ethanol locks have a potential advantage by not creating antimicrobial resistance. Studies of ethanol locks have varied in the ethanol concentrations and luminal dwell times as well as other concurrent catheter care strategies (Majetschak et al. 1999). Several studies have been performed in children receiving home parenteral nutrition; meta-analysis of four retrospective studies in this patient group suggested that ethanol lock therapy decreased the rate of central line-related infections by 81 % (Oliveira et al. 2012). Rarely, occlusion of the central line and catheter-related clots have been described with the use of ethanol locks. Data in children with cancer are lacking (Wolf et al. 2013).
14.2.5.3 Chlorhexidine Cleansing
Chlorhexidine gluconate (CHG) is a bactericidal antiseptic agent that causes membrane disruption. A cloth product with 2 % CHG (Sage Products, Inc., Cary, IL) was approved by the FDA in 2005 for preoperative skin cleansing. Studies of this product in adult intensive care patients have shown a significant decrease in central line infections and acquisition of multidrug-resistant pathogens (Bleasdale et al. 2007; Climo et al. 2009; Popovich et al. 2009). No data are available utilizing this strategy in children with cancer.
14.2.6 Future Directions
Data to evaluate the efficacy and potential toxicity of various strategies for the prevention of serious bacterial infection in pediatric oncology are urgently needed. Use of prophylactic antimicrobial agents needs to be evaluated for efficacy as well as safety in terms of potential short- and longer-term impact on the evolution of bacterial resistance. Studies of levofloxacin prophylaxis (ACCL0934) and CHG cleansing (ACCL1034) in children receiving intensive therapy for cancer are underway. Such research will be critical in understanding the potential merit of various preventative strategies with the ultimate goal of decreasing the burden of bacterial infection and resultant morbidity and mortality in children with cancer. Current recommendations and evidence-based grading for prevention of bacterial infection are summarized in Table 14.1.
14.3 Prevention of Fungal Infections
Children undergoing treatment for cancer are also at increased risk of developing an IFI secondary to breakdown in natural barriers (e.g., indwelling catheter, mucositis), defects in cell-mediated immunity (i.e., lymphopenia from corticosteroids and other anti-T-cell cytotoxic agents), and deficient numbers of phagocytes (due to myelosuppressive chemotherapy) (Lehrnbecher et al. 1997). Having a single defect in the host defense system is often insufficient for development of an opportunistic IFI, but, with multiple defects, IFI begins to emerge as a significant problem. The data on IFI development and potential prevention in immunocompromised hosts derive primarily from adult studies. However, children differ from adults in the types of IFI they develop and in their metabolism of antifungal agents. For example, invasive infections caused by Candida parapsilosis are more common, and Candida glabrata rarer, in children as compared to adults, and invasive aspergillosis (IA) can be more difficult to diagnose in children due to different radiologic findings (Malani et al. 2001, Burgos et al. 2008). Thus, extrapolating clinical decisions from adult trials may be problematic. Once IFI develops, even with the advent of newer antifungal agents, treatment success rates are suboptimal, especially for mold infections. For example, Burgos et al. (2008) found that 53 % of children diagnosed with IA died; therefore, prevention of IFI in pediatric oncology patients is likely most important in improving morbidity and mortality.
14.3.1 Risk Stratification
Based on retrospective reports, as well as toxicity data collected during therapeutic trials, several groups of pediatric patients are at high risk of developing an IFI: patients undergoing HSCT (especially from an alternative allogeneic donor), patients receiving chemotherapy for AML or relapsed ALL and patients with severe aplastic anemia (SAA) (Zaoutis et al. 2006; Burgos et al. 2008). In children being treated for AML, several studies have demonstrated a high incidence (i.e., up to 29 %) of IFI, both in newly diagnosed and relapsed patients (Table 14.2) (Groll et al. 1999; Rosen et al. 2005; Sung et al. 2007, 2009). The high rate of mold infection and secondary morbidity and mortality suggest this group of patients may benefit from anti-mold prophylaxis. Conversely, studies of ALL patients (Table 14.2) suggest that only those with relapsed disease have a high enough incidence of IFI to justify routine prophylaxis (Groll et al. 1999; Leahey et al. 2000; Rosen et al. 2005; Afzal et al. 2009). From a biologic standpoint, patients with relapsed ALL will have generally received years of lympholytic chemotherapy during which time they could have theoretically become colonized with fungal spores which are more likely to become invasive when treated with more aggressive myelosuppressive chemotherapy for their relapsed disease. The risk of IFI in newly diagnosed ALL and solid tumor patients is not high enough to justify routine use of prophylactic antifungals (Rosen et al. 2005; Afzal et al. 2009). A pediatric meta-analysis came to similar conclusions, recommending antifungal prophylaxis in patients with AML/MDS (myelodysplastic syndrome) but not in those with other malignancies even if with anticipated neutropenia >7 days (Science et al. 2014).
Table 14.2
Incidence of invasive fungal infection (IFI) in pediatric oncology patients
Study design | # of patients | Disease/procedure | Prophylaxis agent | IFI incidence | Reference |
|---|---|---|---|---|---|
Prospective (CCG 2961) | 492 | New AML | Nonea | 14–23 % per phase | Sung et al. (2007) |
Prospective (CCG 2891) | 335 | New AML | Nonabsorbablea | 10–27 % per phase | Sung et al. (2009) |
Prospective | 18 | New AML | Fluconazole or nonabsorbable antifungal agent | 29 % | Groll et al. (1999) |
7 | Relapsed AML | 28 % | |||
97 | New ALL | 2 % | |||
35 | Relapsed ALL | 9 % | |||
Retrospective | 261 | ALL | None | 10 % | Rosen et al. (2005) |
117 | AML | 9 % | |||
Retrospective | 425 | New ALL | None | 1 % | Afzal et al. (2009) |
Prospective (CHP-540) | 21 | Relapsed ALL | Fluconazole | 19 % | Leahey et al. (2000) |
14.3.2 Approaches to Antifungal Prophylaxis
Whether antifungal prophylaxis is beneficial in high-risk pediatric cohorts remains controversial due to a lack of sufficient data. Robenshtok et al. (2007) performed a meta-analysis of 64 antifungal prophylaxis trials and demonstrated in patients with acute leukemia that the risk of IFI development was lower with antifungal prophylaxis, yet did not result in a statistical improvement in all-cause mortality. Only 5 of the 64 analyzed trials included pediatric patients making it difficult to generalize to the pediatric oncology cohort. Additionally, data were not collected on potential altered morbidity with IFI prophylaxis such as decreased hospital days or need for intensive care.
Several published randomized prospective trials comparing antifungal agents have included pediatric patients, although rarely younger than 12 years of age, and pediatric patients have generally not been separately analyzed (Table 14.3) (Goodman et al. 1992; Slavin et al. 1995; van Burik et al. 2004; Cornely et al. 2007; Wingard et al. 2010). Therefore, conclusions about the optimal prophylactic agent in pediatric oncology patients are based almost exclusively upon extrapolation from adult data. Currently, the most commonly recommended agent for antifungal prophylaxis in high-risk children is fluconazole although this recommendation is based on two older placebo-controlled trials performed in patients >12 years older of age undergoing autologous or allogeneic HSCT (Goodman et al. 1992; Slavin et al. 1995).
Table 14.3
Selected antifungal prophylaxis trials
Prophylaxis | Design | # of patients (# pediatric) | Disease/procedure | Control outcomea | Intervention outcomea | Reference |
|---|---|---|---|---|---|---|
Fluconazole | DB, PC, MC | 356 (?)b | Auto- and allo-HSCT | 16 % at 50 days (placebo) | 3 % at 50 days | Goodman et al. (1992) |
Fluconazole | DB, PC, SC | 300 (?)b | Auto- and allo-HSCT | 18 % at 75 days (placebo) | 7 % at 75 days | Slavin et al. (1995) |
Amphotericin B | OL, MC | 355 (0) | Auto- and allo-HCT | 9 % (fluconazole) | 14 % | Wolff et al. (2000) |
L-AMB | OL Pilot | 57 (57) | Allo-HSCT | NA | 0 % at 100 daysa | Roman et al. (2008) |
Itraconazole | OL, SC | 304 (5)b | Allo-HSCT | 15 % (fluconazole) | 7 % | Marr et al. (2004) |
Voriconazole | DB, MC | 600 (51) | Allo-HSCT | 11 % (fluconazole) at 6 months | 7 % at 6 months | Wingard et al. (2010) |
Posaconazole | OL, MC | 602 (?)b | AML/MDS | 11 % (fluconazole or itraconazole) at 100 days | 5 % at 100 days | Cornely et al. (2007) |
Micafungin | DB, MC | 882 (84) | Auto- and allo-HSCT | 1.6 % (fluconazole) at 70 days | 2.4 % at 70 days | van Burik et al. (2004) |
Caspofungin | OL, SC | 200 (0) | AML/MDS | 6 % (itraconazole) | 6 % | Mattiuzzi et al. (2006) |
Caspofungin | Retrospective | 123 (0) | Auto- and allo-HSCT | NA | 7.3 % at 100 days | Chou et al. (2007) |
Although it reduced the risk of IFI relative to placebo, fluconazole lacks activity against Aspergillus spp., which is the second most common cause of IFI in these patients. Given this lack of anti-mold activity, several trials have compared it to mold-active agents in hopes of further decreasing rates of IFI. The first of these trials compared fluconazole to low-dose conventional deoxycholate amphotericin B deoxycholate (D-AMB); however, D-AMB did not show improvement over fluconazole and resulted in a higher adverse event rate (Wolff et al. 2000). With the advent of liposomal amphotericin B (L-AMB), there was renewed interest in prophylaxis with an amphotericin B product, and several trials, including one in children, have evaluated L-AMB (often given only three times per week) for antifungal prophylaxis in HSCT and acute leukemia patients (Kelsey et al. 1999; Mattiuzzi et al. 2003; Penack et al. 2006; Roman et al. 2008). Again, like D-AMB, L-AMB has not been shown superior to fluconazole and typically demonstrates increased side effects.
Extended-spectrum azoles such as itraconazole, voriconazole and posaconazole do possess anti-Aspergillus activity (Ashley et al. 2006). Several trials of itraconazole versus fluconazole have been performed and although a meta-analysis showed significantly less IFI, increased side effects, greater drug interactions and poor tolerability have led to itraconazole being abandoned in pediatric patients (Marr et al. 2004; Vardakas et al. 2005). The results of a multicenter, double-blind trial showed that voriconazole was not superior to fluconazole in the prevention of IFI, though the safety profile was similar (Wingard et al. 2010). Given the broader spectrum of activity with voriconazole, this result was surprising, but may have been due to an incomplete understanding of the complex pharmacokinetics of voriconazole and subsequent underdosing. Posaconazole is a triazole with broad coverage of most fungi, including zygomycetes (Ashley et al. 2006). In a randomized, blinded, multicenter trial of AML/MDS patients ≥13 years of age with neutropenia, posaconazole prophylaxis was superior to fluconazole or itraconazole in the prevention of IFI (absolute risk reduction –6 %; 95 % CI, –9.7 % to –2.5 %, p < 0.001), but was also associated with an increased risk of serious adverse events (6 % vs. 2 %, p = 0.01) (Cornely et al. 2007).
The echinocandins (i.e., caspofungin, micafungin, anidulafungin) are a novel class of antifungals that target (1,3)-β-d-glucan synthase and thus interrupt biosynthesis of the glucan polymers that make up fungal cell walls. Because mammalian cells do not possess cell walls, echinocandin administration to patients has resulted in minimal toxicity. Echinocandins possess fungicidal activity against Candida spp. (including Candida krusei and Candida glabrata, which possess significant degrees of fluconazole resistance) and Pneumocystis jiroveci, as well as fungistatic activity against Aspergillus spp. (Ashley et al. 2006). However, they have limited efficacy against Candida parapsilosis. The echinocandins may be superior to fluconazole or amphotericin B for treatment of invasive candidiasis which, when combined with their anti-Aspergillus activity and excellent safety profile, makes them an attractive option for antifungal prophylaxis (Mora-Duarte et al. 2002; Reboli et al. 2007). In a prophylactic trial, micafungin demonstrated reduced need for empiric antifungal therapy and an improved safety profile compared to fluconazole (van Burik et al. 2004). However, the number of pediatric subjects enrolled was small (n = 84), and a reduction in the incidence of proven or probable IFI was not demonstrated. The lack of impact on IFI may have been because the incidence of breakthrough IFI in both groups was very low, likely due to the inclusion of low-risk patients (46 % autologous HSCT recipients) and very few patients undergoing umbilical cord blood transplant (UCBT; n = 30). Caspofungin has been shown to be at least equivalent to itraconazole in the setting of antifungal prophylaxis for adults with AML/MDS with few adverse events (Mattiuzzi et al. 2006; Chou et al. 2007). Similarly, a randomized, blinded, multicenter study of pediatric patients with persistent febrile neutropenia found comparable tolerability, safety and efficacy between caspofungin and L-AMB (Maertens et al. 2010).
14.3.3 Guideline Recommendations for Antifungal Prophylaxis
Most antifungal prophylaxis guidelines are focused on adults with hematologic malignancies or those undergoing HSCT. The IDSA guidelines recommend patients undergoing allogeneic HSCT or intensive remission induction or salvage-induction chemotherapy for acute leukemia to receive anti-Candida agents, with all four azoles, micafungin, and caspofungin as acceptable choices (Freifeld et al. 2011). In patients ≥13 years of age undergoing intensive chemotherapy for AML or MDS, Aspergillus-directed prophylaxis with posaconazole should be considered (Freifeld et al. 2011). Conversely, antiAspergillus agents have not been shown to be beneficial in HSCT recipients unless there is a prior history of IA, anticipated neutropenia (i.e., ANC <0.5 × 109/L) for >2 weeks or a prolonged period of neutropenia pre-HSCT (Freifeld et al. 2011). The European Conference on Infections in Leukemia (ECIL) guidelines are also focused on adult patients undergoing induction chemotherapy or allogeneic HSCT (Maertens et al. 2011). For patients with leukemia, the ECIL guidelines consider posaconazole as having the strongest supportive evidence, with aerosolized L-AMB combined with fluconazole, fluconazole alone, itraconazole and low-dose amphotericin B all having lesser support (Maertens et al. 2011). North American pediatric guidelines strongly recommend fluconazole at a dose of 6–12 mg/kg/day (maximum 400 mg/day) for children with AML/MDS and suggest that posaconazole 200 mg three times daily is an alternative in children ≥13 years of age in settings with a high local incidence of mold infection (Science et al. 2014). In contrast, pediatric ECIL guidelines suggest either posaconazole in children ≥13 years or itraconazole in those >2 years as a recommendation with moderate evidence in patients with high-risk ALL, AML or relapsed leukemia (Groll et al. 2014).
14.3.4 Limitations of Current Options for Antifungal Prophylaxis
The current options for antifungal prophylaxis all have certain limitations: fluconazole is generally well tolerated, but has a limited spectrum of activity that does not include invasive molds; itraconazole is poorly tolerated due to gastrointestinal side effects; voriconazole, though an attractive option in children >12 years of age (age of most trial data), has questionable standard dosing regimens and multiple drug interactions; posaconazole lacks pharmacokinetic data in children <13 years of age, lacks an intravenous formulation, has unreliable absorption in the setting of limited oral intake, and shares many of the same enzymatic pathways and therefore drug interactions as voriconazole; and finally, echinocandins are expensive and lack an available oral formulation.
For voriconazole in particular, relatively well-established dosing regimens exist for children and adults although recent studies have questioned these standard dosing regimens and have instead proposed dosing based on serum drug levels although the optimal serum voriconazole level is still uncertain (Smith et al. 2006; Trifilio et al. 2007). Part of this variability may be due to allelic polymorphisms of the gene encoding CYP2C19, which can result in an increase or decrease in voriconazole metabolism (Pascual et al. 2008). In children the situation is further complicated by linear kinetics; the optimal dose may be 7 mg/kg twice daily for children from 2 to 12 years of age, while in children <2 years of age it may be as high as 8.5 mg/kg twice daily (Karlsson et al. 2009; Neely et al. 2010; Shima et al. 2010). Even more recent data has led to a proposed maintenance dose of 8 mg/kg twice daily for all children <12 years of age and for those 12–14 years of age weighing <50 kg (Friberg et al. 2012). Currently there is no universally accepted approach to dosing or monitoring of serum levels. Voriconazole also has significant drug interactions with commonly used agents in pediatric oncology patients: voriconazole is a substrate of CYP2CP (major), 2C19 (major), and 3A4 (minor) and an inhibitor of 2C9 (moderate), 2C19 (weak), and 3A4 (moderate) (Cronin and Chandrasekar 2010). Proton pump inhibitors increase voriconazole levels, while voriconazole increases serum levels and toxicity of corticosteroids, vincristine, imatinib, bortezomib, irinotecan and many other medications (Cronin and Chandrasekar 2010).
14.3.5 Risks of Prophylaxis
In addition to direct toxicities (such as renal or hepatic) and medication interactions (especially with azoles), utilization of an antifungal agent can induce selective pressure and lead to the development of resistant organisms. Resistance of various Candida spp. to fluconazole is a well-known phenomenon and recently echinocandin resistance has also been noted (Pfaller et al. 2012). There is also concern that more widespread usage of prophylactic voriconazole has led to more cases of Mucorales infection (Trifilio et al. 2007). Theoretically this may be due to competitive inhibition such that Mucorales will not invade if Aspergillus spp. are present, but with voriconazole inhibition of Aspergillus, the less common Mucorales will find an opportunity to invade. In vitro data also suggest that voriconazole directly increases the virulence of zygomycetes (Lamaris et al. 2009).
14.3.6 Biomarkers
The European Organisation for Research and Treatment of Cancer and Mycoses Study Group (EORTC/MSG) has established guidelines to standardize the definitions of proven, probable and possible IFI (De Pauw et al. 2008). However, in practice, the diagnosis of IFI is often difficult because of the lack of specific symptoms and the invasiveness of standard diagnostic tests. Significant attention has been focused on developing noninvasive assays such as galactomannan (GMN) and (1,3)-β-d-glucan (BDG) to diagnose IFI and as discussed in Chap. 1. GMN is a polysaccharide cell wall component that is released by Aspergillus during growth and BDG is a cell wall polymer found in all fungi except Cryptococcus spp. and zygomycetes. Although commercially available kits for detection of both GMN and BDG are approved by the FDA for adults, the role of GMN in diagnosing IFI in the pediatric population remains undefined and data on BDG testing in pediatric patients are extremely limited (Steinbach et al. 2007). Until further research on these and other noninvasive tests is performed, the potential for early diagnosis of IFI in pediatric oncology patients remains elusive.
14.3.7 Future Directions
The profound lack of data for this patient population have led to a clinical situation where there is no clear agent of choice for patients at high risk of developing an IFI. Because of this, in April 2011 the COG initiated a randomized open-label trial of caspofungin compared to fluconazole to prevent IFI in children undergoing chemotherapy for AML. As noted previously, a number of therapy-induced alterations in host defense have been identified as risk factors for IFI. However, there is also considerable emerging evidence that a genetic component exists in the susceptibility and outcome of IFI for immunocompromised populations. In allogeneic HSCT recipients, several polymorphisms in both host and donor genes appear to significantly predispose patients to IFI (Table 14.4) (Kesh et al. 2005; Seo et al. 2005; Granell et al. 2006; Bochud et al. 2008; Mezger et al. 2008; Zaas et al. 2008; Cunha et al. 2010). Future investigation will likely uncover additional polymorphisms that place immunocompromised hosts at increased risk of IFI. As more details on genetic risk factors emerge and are validated, personalized risk stratification will be improved beyond the current system that only utilizes traditional IFI risk factors. Furthermore, although all such studies to date have been performed in allogeneic HSCT patients, biologically it is reasonable to assume that these polymorphisms will also play a role in the development of IFI during treatment of AML, relapsed ALL, SAA and, potentially, even lower-risk diseases. Current recommendations and evidence grading for prevention of IFI are summarized in Table 14.5.
Table 14.4
Genetic risk factors for development of invasive fungal infection (IFI) following allogeneic hematopoietic stem cell transplant (HSCT)
Infection | Gene | Source | # of HSCTs | Hypothetical mechanism | Reference |
|---|---|---|---|---|---|
IA | TLR1 and TLR6 | Host | 127 | Decreased recognition by phagocytes | Kesh et al. (2005) |
IA | IL-10 promoter | Host | 105 | Less production of IL-10 | Seo et al. (2005)
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|