History
Parameter
Action
Age/family history
Obesity
BMI
Avoid further gain, gradual loss
Diabetes
HbA1C/Fasting glucose
Initiate diabetic therapy
Inactivity
Minutes of recreational physical activity/week
Encourage 150 min or more recreational physical activity per week
Fitness
VO2 peak/6 min walk
Gradual increase with exercise to moderately fit or better
Hypertension
BP
Monitor/correct
Dyslipidemia
Fasting lipid profile
Initiate therapy
Smoking
Smoking cessation aids
Cardiac evaluation
LVEF:
Echocardiogram or MUGA
Avoid cardio-toxic drugs if abnormal
Referral to cardiologist
Cardiac evaluation
EKG/QT interval
Avoid cardio-toxic drugs if abnormal
Referral to cardiologist
Development of simple evidence based oncology guidelines for the optimal screening of asymptomatic women for cardiac risk factors, and timing of initiation of treatment for reversible conditions should have major importance in the survivorship research agenda. This is will take a collaborative effort between Medical Oncologists, Cardiologists and Primary Care Physicians. The International CardiOncology Society (ICOS) was founded to review emerging trial evidence and make recommendations to assess risk and provide treatment recommendations for patients undergoing treatment for a variety of cancers (Lenihan et al. 2010).
Need for Use of Standard Nomenclature to Describe Risk and Severity of Cardiac Dysfunction
The increase in rates of clinical heart failure in pivotal trials of trastuzumab in women with metastatic disease, especially those who were receiving or who had recently received anthracyclines, prompted establishment of the Cardiac Review and Evaluation Committee or CREC. Initial CREC criteria used to describe cardiac dysfunction and subsequently employed in many adjuvant trials were (1) cardiomyopathy characterized by a global decrease in left ventricular ejection fraction (LVEF); (2) signs or symptoms of congestive heart failure (CHF); (3) decline in LVEF of at least 5 % to less than 55 % with signs or symptoms of CHF; or (4) decline in LVEF of at least 10 % to less than 55 % without signs or symptoms of CHF (Seidman et al. 2002). The definition of cardiac dysfunction in oncology trials has differed somewhat from trial to trial making cross trial comparisons somewhat difficult. For example a drop in LVEF by 10 points or more to less than 55 % was considered evidence of cardiac dysfunction in the NSABP B-31 trial but the HERA trial required an absolute drop of 10 points or more to an LVEF of less than 50 % (Tan-Chiu et al. 2005; Piccart-Gebhart et al. 2005). Research in cardiac dysfunction/heart failure in breast cancer survivors would be facilitated by agreement on standard terminology for assessment in clinical trials. Use of American Heart Association criteria for heart failure is appropriate: Stage A risk factors present but no structural damage; Stage B structural damage but no signs or symptoms; Stage C structural damage with current or prior symptoms; and Stage D structural damage with symptoms with any physical activity or at rest (Yancy et al. 2013) (see Table 14.2). Asymptomatic reduction in left ventricular ejection fraction (LVEF) by 10 or more points to <50 % is generally viewed as representative of the Stage A to Stage B transition and is associated with a significant increase in cardiac mortality (Lenihan et al. 2013). Consequently, an asymptomatic reduction in left ventricular ejection fraction by 10 or more points to <50 % has come to represent a surrogate endpoint for cardiac injury in clinical trials and in the remainder of this manuscript will be referred to as subclinical cardiac toxicity and/or cardiac dysfunction.
Table 14.2
American Heart Association criteria for heart failure (Yancy et al. 2013) and treatment by stage
AHA heart failure stage | New York Heart Association | Current Treatment Recommendations (Rx) | Research needed |
|---|---|---|---|
Stage A: High risk but no structural damage | I | Correct risk factors: hypertension, hyperlipidemia, diabetes, obesity. poor fitness, alcohol and smoking | Predictive model of transition A to B Protective Rx to prevent A to B transition Application research |
Stage B: Structural damage but no signs or symptoms of heart failure (LVEF < 50 % but usually >40–45 %) | I | Correct risk factors B blockers, ACEI or ARBs as appropriate | Sensitive monitoring tools detect A to B transition Rx to allow cardiotoxic drugs |
Stage C: Structural heart disease. prior or current symptoms (LVEF <40 %) | I-IV | Discontinue cardiotoxic drug Heart failure therapy | Rx to allow re-institution of cardiotoxic drugs once compensated |
Stage D: Symptoms at rest or any physical activity | IV | Heart failure therapy | N/A |
Drug Related Cardiac Toxicity
Although many drugs can result in cardiac damage (Yeh et al. 2004; Bovelli et al. 2010), we will focus here on the two most commonly used types of agents with the greatest potential for cardiac dysfunction, namely anthracyclines and HER-2 targeted agents.
Anthracyclines
Anthracyclines increase free radical formation, mitochondrial oxidative stress, disruption of myofibrils, apoptosis of cardiomyocytes, and reduction of the cardiac stem cell pool (Kumar et al. 1999; Zhang et al. 2012; De Angelis et al. 2010). A recent meta-analysis suggests that without regard to dose, treatment with anthracyclines increases clinical cardiac toxicity, usually manifested as heart failure, by approximately fivefold, subclinical toxicity by sixfold, and the risk of cardiac death by fivefold (Smith et al. 2010). The absolute risk of clinical or subclinical cardiac toxicity depends upon the type of anthracycline, the cumulative dose, exposure to additional cardiotoxic agents, patient age, African American race, and other co-morbidities (Bowles et al. 2012; Lotrionte et al. 2013; Perez et al. 2004; Bird and Swain 2008). By the time significant changes are noted in LVEF (drop of 10 or more points to <50 %) permanent damage has generally already occurred.
Clinical heart failure is very rare in healthy individuals taking a cumulative doxorubicin dose of 240 mg/m2 (the average with four cycles of doxorubicin and cyclophosphamide) unless other cardiotoxic agents are being administered concomitantly; but subclinical cardiac toxicity (an asymptomatic decrease in LVEF by 10 or more points to <50 %) was reported in 3.3 % of healthy women with a median age of 52 receiving four cycles of this anthracycline based regimen with short follow-up (Perez et al. 2004). A recent review of 18 studies with over 20,000 women treated with anthracyclines at varying cumulative doses and followed for a median of 9 years, reports an asymptomatic drop in LVEF to <50 % occurred in 18 %; and symptomatic cardiac toxicity, primarily heart failure, occurred in 6 % (Lotrionte et al. 2013). Clinical heart failure in the 40 % of women with breast cancer over 65 treated with anthracyclines is substantially higher due in large part to the underlying presence of asymptomatic heart disease. Using data from a large SEER data base, 38 % of women age 66–70 at the time they took anthracycline-based chemotherapy had developed congestive heart failure 10 years later compared to 32 % of women taking non-anthracycline chemotherapy and 29 % of women not taking any chemotherapy (Pinder et al. 2007).
Epirubicin is ~60 % less likely to result in cardiac toxicity than doxorubicin and liposomal doxorubicin has a 22 % lower risk than doxorubicin. Finally, continuous infusion doxorubicin over several days has approximately one-fourth of the cardiac toxicity as bolus doxorubicin (Smith et al. 2010).
The substantially higher rates of congestive failure in women over 65 taking bolus doxorubicin suggests that most older women should receive either non-anthracycline containing regimens, less toxic forms of anthracyclines, or protective therapy. Continuing research in this are using sensitive biomarkers to predict a drop in left ventricular ejection fraction area the abnormal range with early intervention prior to drop in ejection fraction is warranted (see below).
Inhibition of topo-isomerase II DNA binding appears to be an important factor in both anthracycline induced tumor cell death and cardiomyocyte injury. However, recent evidence suggests that TOP2A may be the primary target for doxorubicin induced tumor cell death whereas TOP2B may be the primary intermediate in cardiac induced injury (Vejpongsa and Yeh 2014). These data suggest that development of TOP2A specific anthracyclines may be an important future area of research (Sawyer 2013).
Trastuzumab and Other HER-2 Targeted Agents
The epidermal growth factor receptors are involved in proliferation and regeneration, metabolism, differentiation and cell survival. HER-2 (ErbB2) is one of four epidermal growth factor receptors expressed on the surface of cardiomyocytes as well as breast cancer cells. Hetero- or homo-dimerization of receptors is necessary for downstream signaling. ErbB2 has no identified natural ligand but is the preferred binding partner for the other ErbB receptors. Dimerization of HER-2 can be induced by an increase in receptor concentration or by ligand binding (EGF, TGF alpha and amphiregulin for ErbB1 and neuregulin for ErbB3/4) with one of the other ErbB receptors. Homo-dimerization of ERbB2 or hetero-dimerization of ErB2 and ErbB3 in HER-2 amplified breast cancer leads to a dramatic increase in proliferative and survival signals primarily through the PI3 kinase pathway (De Keulenaer et al. 2010). ErbB2 is expressed at low levels in the adult heart and is up regulated in response to stress or injury (such as with anthracyclines) (De Keulenaer et al. 2010). Neuregulin is released by endothelial cells and once bound to ErbB4 results in hetero-dimerization with ErbB2 and proliferation of cardiac progenitor cells and possibly de-differentiation and proliferation of differentiated cardiomyocytes (De Keulenaer et al. 2010; Hervent et al. 2012). Trastuzumab binds with ErB2, disrupting the Neuregulin 1β /ErB2/ErbB4 complex which in turn prevents proliferation of cardiac progenitor cells in response to stress (Bersell et al. 2009; Fedele et al. 2012). Investigators are looking at rational designs for HER-2 targeted agents which will not disrupt the neuregulin/ErbB4 complex (Fedele et al. 2012). Use of neuregulin to prevent or treat preclinical cardiac damage is also of interest (Bersell et al. 2009).
Trastuzumab without prior anthracyclines or other underlying cardiac risk factors may be associated with declines in LVEF but generally not irreversible heart failure. However, both asymptomatic cardiac dysfunction and symptomatic heart failure occur more frequently in individuals given trastuzumab after or concomitantly with an anthracycline compared to an anthracycline alone (De Keulenaer et al. 2010; Perez et al. 2008; Gianni et al. 2011; Goldhirsch et al. 2013). Utilizing CREC criteria, trastuzumab with a taxane in women with prior anthracycline exposure had a cardiac dysfunction rate of 13–16 % and a New York Heart Association class III or IV heart failure rate of 2–4 % whereas trastuzumab used concomitantly with anthracyclines was associated with a cardiac dysfunction rate of ~27 % and a New York Heart Association class III or IV heart failure rate of ~16 % in early trials (Seidman et al. 2002). A recent update of the Herceptin Adjuvant or HERA trial in which one or 2 years of trastuzumab was given after adjuvant therapy, cardiac adverse event leading to discontinuation occurred in 9.4 % of women on the 2 years arm vs. 5.2 % of women on the 1 year arm. All but 12–20 % of women with significant LVEF declines recovered with less than 1 % developing symptomatic congestive heart failure (de Azambuja et al. 2014). Risk factors for cardiac dysfunction in addition to anthracyclines for women receiving adjuvant trastuzumab are age >60, a borderline normal left ventricular ejection fraction (50–55 %) at baseline and pre-existing hypertension (Tan-Chiu et al. 2005). Despite theoretical concerns, using two HER-2 targeted agents simultaneously such as pertuzumab and trastuzumab or lapitinib and trastuzumab does not appear to increase cardiac toxicity to a greater extent than trastuzumab alone, although there is limited long term experience (Valachis et al. 2013; Baselga et al. 2012).
Older women are likely to have one or more cardiac risk factors at baseline and were under-represented in clinical trials. An analysis from a Medicare claims data base of women 67 and older with early breast cancer indicates that 32 % had evidence of cardiac dysfunction or heart failure if they took trastuzumab alone, 42 % if they received both trastuzumab and anthracyclines but only 18 % of those receiving no adjuvant therapy (Vaz-Luis et al. 2014). Another population based study of women >65 most of whom took their trastuzumab concomitantly with chemotherapy noted a 3.6 % hospitalization rate for cardiac events during treatment (Chen et al. 2012) Research with cardio-protective regimens coupled with biomarkers of early injury is needed targeting older women receiving trastuzumab or others with baseline risk factors for cardiac dysfunction (Wells and Lenihan 2010).
Physical Activity and Cardiorespiratory Fitness
Although, oncologists are increasingly aware of the potential adverse effects of excessive weight on breast cancer outcomes and mortality, there has not been the same emphasis on physical activity and cardiorespiratory fitness.
Physical activity is associated with lower cardiovascular, all cause and breast cancer mortality (George et al. 2011; Irwin et al. 2011; Dhaliwal et al. 2013). In the Women’s Health Initiative 9 MET hours or about 3 h of fast walking per week pre or post diagnosis of breast cancer was enough to see an ~40 % decrease in breast cancer and all-cause mortality compared with sedentary women (Irwin et al. 2011). The important message then for women is to become active even if they were not prior to their diagnosis of breast cancer.
Cardiorespiratory fitness as measured by oxygen consumption at peak exercise (VO2max or VO2peak) is correlated with cardiovascular and all-cause mortality (Peel et al. 2009). In a general population increasing fitness by only one metabolic equivalent (1 MET corresponds to 3.5 mL/min/kg of oxygen consumption) is estimated to reduce risk of death by 13 % (Kodama et al. 2009). Individuals with low CRF (<7.9 METs) had a 40 % increase in all-cause mortality and 47 % increase in risk for cardiovascular events compared with those with intermediate CRF (7.9–10.8 METs). Women with low CRF had a 70 % increase in all-cause mortality and 56 % increase in cardiovascular events compared to those with high CRF (≥10.9 METs) (Kodama et al. 2009). Further, fitness may be a more important predictor of mortality than Body Mass Index (BMI) until one reaches the extremes of obesity where serious metabolic abnormalities are prevalent. In a recent meta-analysis, fit overweight and obese women had similar mortality as fit normal weight women, and were generally better off than normal weight unfit women unless the obese fit woman also had a chronic disease (Barry et al. 2014). 150 min of moderate physical activity per week or that sufficient to expend at least 1,000 Kcal per week is likely to allow most women to attain at least the lower bound of the intermediate CRF category (Lee and Skerrett 2001).
Women with breast cancer appear to have lower baseline CR fitness than age-matched individuals without cancer and fitness declines during treatment (Jones et al. 2012; Peel et al. 2014). Low fitness was reported in approximately half of women who had undergone chemotherapy with anthracyclines + taxanes with or without trastuzumab ~2 years after chemotherapy completion despite a normal LVEF in all but 8 % (Jones et al. 2007). These rates were dramatically higher than age-matched controls who had not received chemotherapy (Jones et al. 2007).
Research efforts need to focus on effective interventions which will prevent reduction of physical activity and fitness during and after treatment. Courneya et al. using a supervised aerobic and strength training exercise program have demonstrated that higher volume exercise can be safely delivered during adjuvant treatment, which in turn appears correlated with improved disease free and overall survival (Courneya et al. 2013, 2014). Higher intensity exercise may also favorably modulate pro-inflammatory biomarkers associated with cardiovascular disease risk (Fairey et al. 2005).
Methods to safely increase physical activity and fitness in a more practical, home-based environment following initial in-person training need to be a research priority, particularly for older women and those with a low level of fitness at baseline. Preliminary pilot studies suggest this is possible (Burnett et al. 2013). Further, simplified methods of assessing fitness which can easily be employed in an oncologist’s office such as the 6 min step test need to be validated against more complex tools such as VO2max or VO2peak (Hamilton and Haennel 2000; Simonsick et al. 2006).
Risk Prediction Models for Cardiac Toxicity
Prediction models in older women at highest risk for cardiac toxicity have been suggested using variables of age, type of adjuvant therapy, and prior history of hypertension, diabetes, and symptomatic heart disease (Ezaz et al. 2014). Research is needed to develop more sensitive and specific cardiac dysfunction prediction models for women considering potentially cardiotoxic therapy which would include risk biomarkers and historical variables. These prediction models would ideally have the ability to include some of the new very sensitive indicators of left ventricular function (Fallah-Rad et al. 2011), in addition to weighted factor scores for age, BMI, a measure of physical activity or cardiorespiratory fitness, hypertension, diabetes, dyslipidemia, and any prior cardiac event. Ideally the models could also be adapted to include provision for genetic polymorphisms predisposing to cardiac risk such as the common HER-2 Ile655Val allele in the HER-2 gene (Beauclair et al. 2007), and polymorphisms in NADPH oxidase, MDR 1, MDR 2, catalase, superoxide dismutase, NADPH:quinone oxidoreductase, carbonyl reductase 3, and glutathione s-transferase (Wojnowski et al. 2005; Deng and Wojnowski 2007).
Monitoring Cardiac Function During Treatment with Cardiotoxic Agents
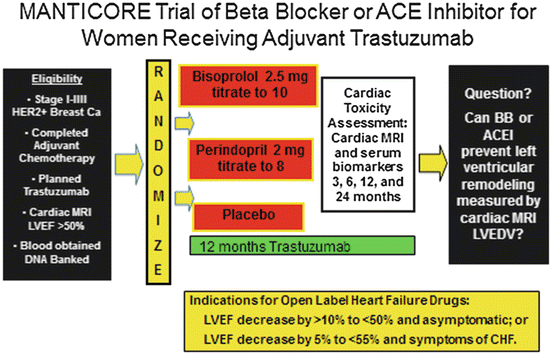
Cardiac MRI is a sensitive method for detecting left ventricular remodeling and early subclinical cardiac toxicity and is considered by some to be the gold standard (Fallah-Rad et al. 2011). It is currently being used in the MANTICORE trial to measure left ventricular end diastolic volumes (see below). However, cardiac MRI is expensive and thus not optimal for serial monitoring in the clinical community setting (Bellenger et al. 2000). Longitudinal or global left ventricular strain, which can be calculated from an echocardiogram with the appropriate software, is a measure of cardiac muscle deformability. The greater the negative value the more powerful the contraction. Normal strain values vary with age, sex and the software system used from approximately −15 to −22 (Yingchoncharoen et al. 2013; Cheng et al. 2013), but a value of −19 or more negative is highly unlikely to be associated with cardiac dysfunction or clinical congestive heart failure over the ensuing 3 months of cardiotoxic treatment (Sawaya et al. 2011, 2012). Adding a serum troponin drawn before and immediately after chemotherapy to left ventricular strain is reported to improve both sensitivity and negative predictive value for left ventricular dysfunction after anthracyclines (Fallah-Rad et al. 2011; Cardinale et al. 2010). Although some studies have suggested additional serum markers such as and high sensitivity c-reactive protein (hsCRP) may aid in predicting cardiac dysfunction, others have not found that the addition of serum biomarkers in addition to troponin to substantially increase sensitivity (Onitilo et al. 2012), in 78 patients receiving both doxorubicin and trastuzumab, found the addition of hsCRP and BNP did not add additional predictive information to ultrasensitive troponin and myeloperoxidase in predicting dysfunction by CREC criteria (Ky et al. 2014). 3D echocardiography may be more sensitive than 2D to declines in left ventricular function (Khouri et al. 2014).
Studies incorporating one or more of these markers into the monitoring process for cardiac toxicity (i.e., PREDICT trial) are currently ongoing. Left ventricular strain has perhaps the greatest potential in that the software is relatively easy to add to an echocardiogram machine and is a non-invasive procedure. Unfortunately, there are variations in the software and interpretations of results such that at present there is not a clear indication as to what constitutes a definitely abnormal strain or the amount of change which should be viewed with alarm.
Trials comparing the relative efficacy of newer sensitive biomarkers of subclinical injury prior to significant decline in 2D echo left ventricular ejection fraction, including high sensitivity troponin and other serum biomarkers, left ventricular strain, and cardiac MRI are ongoing and results are awaited with interest. Translational trials incorporating prophylactic treatment with beta blockers and angiotensin converting enzyme inhibitors at first signs of subclinical dysfunction are warranted.
Treatment of Cardiac Dysfunction
Beta blockers, angiotensin converting enzyme inhibitors (ACEI), and angiotensin receptor blockers (ARBs) are commonly used to treat heart failure regardless of the inciting event. They are recommended in the general cardiovascular setting for asymptomatic women with a drop in left ventricular ejection fraction below 40 % or women with symptomatic congestive heart failure regardless of the amount of decline in LVEF (Yancy et al. 2013). Patients who develop symptomatic cardiac dysfunction while on treatment with a cardiotoxic agent should be treated as would anyone not on chemotherapy (Yeh et al. 2004). For women with early breast cancer, treatment with the cardiotoxic agent should be discontinued and non-cardiotoxic therapy substituted where possible. For women with metastatic HER-2 amplified breast cancer, temporarily discontinuing HER-2 targeted agents, treatment with blockers and ACEIs and then cautious re-introduction of the HER-2 targeted agent is often successful with continued indefinite use of the beta blocker and ACE inhibitor (Vaz-Luis et al. 2014).
What about asymptomatic women with subclinical cardiac toxicity whose LVEF is below 50 % but above 40 %? From the general population experience, it is clear that individuals with an asymptomatic ejection fraction below 50 % have a ~3.5-fold increase in all- cause mortality over the ensuing 9–10 years (Yeboah et al. 2012a). Use of ACEI and beta blockers in women with LVEF <50 %, and appropriate surgical treatment of heart valve problems resulting from radiation or medical or surgical treatment of coronary artery blockage is suggested (Lenihan et al. 2013; Lenihan 2012).
Prophylactic Treatment with Cardioprotective Drugs
An area gaining momentum is the use of prophylactic drugs to reduce the incidence of asymptomatic left ventricular dysfunction (Swain and Vici 2004; van Dalen et al. 2008), particularly if early warning biomarkers can be used to trigger the use of the agent. Prophylactic cardioprotective therapy is not a new concept. A decade ago Swain et al. published a study suggesting doxorubicin induced cardiac toxicity can be reduced ~70 % by also giving the iron chelating agent dexrazoxane (Smith et al. 2010; Swain and Vici 2004). The current recommendation is that dexrazoxane can be given prophylactically once the cumulative doxorubicin dose exceeds 300 mg/m2 (Yeh et al. 2004). Dexrazoxane is not widely used in clinical practice due to concerns that it may reduce effectiveness of doxorubicin as it binds to both TOPO 2A and TOPO 2B (Sawyer 2013).
Since individuals with a low LVEF, even if asymptomatic, have an increased risk of cardiac events and mortality, prophylactic cardio-preventive treatment trials are ongoing in the adjuvant setting of HER-2 positive breast cancer. Several small trials have shown cardioprotective effects of ACEI or beta blockers, especially when given with anthracyclines (Kalay et al. 2006; Bosch et al. 2011). Adjuvant trials with endpoints of symptomatic heart failure or a 2D echo measured LVEF of <50 % would require a very large number of participants. An alternative approach is to use a more sensitive indicator of early cardiac dysfunction. In the MANTICORE trial (Fig. 14.1) women taking trastuzumab are randomized to prophylactic cardiac protective therapy with beta blockers and/or ACEI but the primary outcome is change in left ventricular end diastolic volume as measured by cardiac MRI (Pituskin et al. 2011). With this endpoint as opposed to 2D echo LVEF, the trial coordinators are predicting that 158 randomized subjects will be adequate. Another approach to assess the benefit of prophylactic therapy is to use the traditional endpoint of LVEF drop by 10 points to <50 % but with a much higher risk group as the cohort, such as women with HER-2 amplified metastatic disease. Since these women have generally been previously treated with cardiotoxic drugs and since survival is dependent on being able to continue to receive HER-2 targeted therapy after first progression, the study question is of practical importance (Extra et al. 2010). Such a trial has been proposed in the Southwest Oncology Group Survivorship Committee in women with first and second line HER-2 amplified metastatic disease on HER targeted therapy with LVEF >50 % at baseline to determine whether prophylactic beta blocker therapy with Carvedilol will reduce cardiac dysfunction (See Fig. 14.2). Other target populations for prophylactic cardio-protective therapy where the event rate is likely to be high in either the metastatic or adjuvant setting are women 70 or over, high coronary artery calcium, and hypertension, or a high score on a CVD risk prediction model (Yeboah et al. 2012b).