Fig. 3.1
The 5-year overall survival rate in patients with ulcerative colitis–colorectal cancer (UC-CRC). The 5-year overall survival rate was 89 % (Citation from Ref. [15])
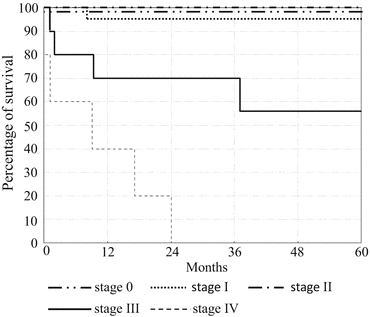
Fig. 3.2
The 5-year survival rates of patients with ulcerative colitis at each stage. Stages 0–II, 100 %; stage I, 96 %; stage III, 56 %; and stage IV, 0 % (Citation from Ref. [15])
3.3 Prognosis of Patients with CD and CA-CRC
Unlike in UC, the risk and prognosis of CA-CRC in CD patients are less well validated. Previous studies on the risk of intestinal cancer in CD reported inconsistent results, with relative risk estimates ranging from 0.8 to 20.0 [49]. Previous studies concluded that the predictors of CA-CRC in CD must be evaluated based on the intestinal segment(s) involved by CD, given the different disease behaviors and thus the potential for cancer in the small bowel, colorectal, or anal region.
The risk of CRC in long-standing CD involving the colon is probably comparable to that of UC [2, 3, 50–55]. In a Danish nationwide cohort study, although the relative risk (RR) of CRC in IBD was low in the first years after diagnosis, it was significantly higher than in the background population after a disease duration of 13 years, reaching 50 % [56]. These results are also consistent with the current surveillance guidelines of the American Gastroenterological Association [5] and the British Society for Gastroenterology [57], both of which recommend the initiation of surveillance after 8–10 years of disease duration for CD, as for extensive UC. However, up to a third of IBD patients develop CRC prior to the initial surveillance colonoscopy [56, 58, 59], suggesting the need to reconsider the present surveillance strategy. An additional complicating factor is that the majority of patients with extended colonic CD lesions may require colectomy after a shorter disease duration than is the case in UC patients.
A meta-analysis from 2005 by Jess et al. [60] limited to population-based studies estimated a pooled overall standardized incidence ratio (SIR) for CRC in CD patients of 1.9 [95 % confidence interval (CI), 1.4–2.5]. Separate risk estimates for cancer in the colon and rectum resulted in a significantly higher risk for colon cancer (SIR = 2.5; 95 % CI, 1.7–3.5), while there was no significantly increased pooled risk for rectal cancer (SIR = 1.4; 95 % CI, 0.8–2.6). The risk of CRC was significantly higher in CD patients with colonic involvement (SIR = 4.3; 95 % CI, 2.0–9.4).
The incidence of CRC in CD is similar in Japan and Western countries, but the anatomic location of CRC differs between the respective populations. The study of Jess et al. [60] included populations from North America, Scandinavia, and Israel. Yano et al. [61] found that the SIR for CRC in Japanese patients with CD was 2.79 (95 % CI, 1.28–5.29); in that series, eight of nine patients with CD and CRC had anorectal cancer (ARC). Mizushima et al. [62] also found that the risk of CRC was significantly higher in patients with CD than in the general population (SIR = 5.80; 95 % CI, 2.13–12.68); in their series, five of six patients with CD had ARC. In a review of the Japanese literature, Sugita et al. [63] reported a high incidence (55–89 %) of ARC in patients with CD and CRC. A noteworthy feature of that study was the finding that 55–68 % of the ARC cases involved a colonic stricture without a fistula. In CD, ARC can arise at a similar (or higher) rate in patients with strictures as in those with fistulas. The anatomic location of CRC in CD patients may show racial differences, as suggested by the higher incidence of colonic involvement in Western countries and a higher incidence of anorectal involvement in Asian countries.
With respect to ARC in CD, despite the lack of population-based studies specifically evaluating ARC and although previous studies were from Western countries, it seems that malignancy rarely arises in perianal fistulas [64, 65], but cancer can certainly arise from a long-standing perianal CD fistula [66–68]. Iesalnieks et al. [69] reviewed 23 published reports describing 59 CD patients with adenocarcinoma arising from perianal fistulas. The authors noted that, while the exact pathogenesis of ARC is unclear, the risk of malignant transformation is significantly higher in patients with fistulizing inflammation. They also observed that adenocarcinomas associated with anal fistulas in patients without CD seem to share many features of ARC in those with CD, including a long-term history of perianal fistula (10–20 years) and an increased incidence of mucin-producing adenocarcinomas. Thus, the presence of a long-standing fistula, rather than the disease behaviors of CD itself, seems to be an important predisposing factor for ARC.
Cancer surveillance can be difficult in patients with CD regardless of the anatomic location of either CRC or ARC, because the thoroughness of the examination may be limited by the presence of strictures. Ky et al. [68] suggested that, especially in ARC, carcinoma arising in a CD fistula is very difficult to diagnose, because the examination for anorectal lesions may be limited by pain, stricture, or induration of the perianal and perineal tissues. They noted that although examination under anesthesia can also overlook the lesion, it generally increases the yield of biopsies or curettage of the fistulous tracts. Examination under anesthesia in these cases is also recommended in Japan, but its efficacy has yet to be confirmed [63].
Regardless of differences in the anatomic locations of CRC in different populations, the prognosis of CRC is worse in CD patients than in UC patients. A nationwide population-based cohort study in Denmark [70] established that CD patients with CRC had a significantly worse mortality. Of patients with a history of CD, 62 % died within the first 5 years compared with 56 % of the matched cohort consisting of patients with CRC but without IBD. For CRC patients with CD, the corresponding mortality ratios compared with patients with non-IBD CRC were 1.33 (95 % CI, 1.06–1.66) and 1.26 (95 % CI, 1.07–1.49).
The prognosis of patients with CD and ARC is poor. Igors et al. [69] reported the survival data of 59 CD patients with ARC. The overall survival rates after proctectomy were 88 % at 1 year, 54 % at 3 years, and 26 % at 5 years. Perirectal lymph node metastases were significantly associated with poor outcome (P < 0.0001), and all patients with lymph node metastases (n = 15) died within 40 months after proctectomy. In a review of the Japanese literature, the overall survival rate after proctectomy was similar: 45 % at 5 years and 30 % at 10 years, even in patients who received postoperative chemotherapy. The overall 5-year survival rate of CA-CRC in 32 patients with CD at our institution between January 1984 and April 2014 was 39.3 % (Fig. 3.3). The patients included three with stage I, 16 with stage II, and six with stage III disease as well as seven patients with an unclassified advanced stage of cancer.
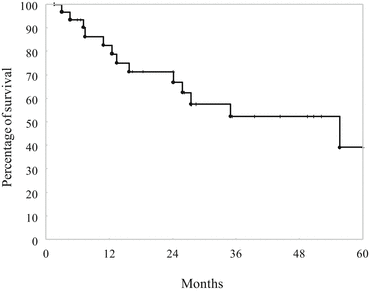

Fig. 3.3
The 5-year overall survival rate of intestinal cancer in patients with Crohn’s disease. The overall 5-year survival rate in 32 patients was 39.3 % (Unpublished data at our institution)
Small bowel carcinoma (SBC) in patients with CD has been discussed controversially. SBC in patients with small bowel CD has long been viewed as a rare and ominous disease for which any form of screening or early detection strategy is unlikely to be useful [49]. A meta-analysis from 2005 by Jess et al. [60] found that, although the pooled overall SIR for CRC in CD patients was 1.9 (95 % CI, 1.4–2.5), the SIRs of SBC were not increased either in patients with ileocolonic CD (SIR = 2.6; 95 % CI, 0.8–8.2) or in those with pure jejunoileal disease (SIR = 0.9; 95 % CI, 0.2–4.1). In the meta-analysis of Canavan et al. [49], also published in 2005, analyses of site-specific CD showed no increase in the RR of SBC in the subset of CD patients with ileal disease (RR = 1.1; 95 % CI, 0.8–1.5).
In five previous population-based meta-analyses, the SIR of SBC ranged from 18.75 to 33.2 in patients with CD [49, 60, 70–72]. Jess et al. [60] found that the number of cases of SBC in patients with CD of the small bowel (nine in 26,780 patient-years) was similar to that of CRC in patients with CD of the colon (ten in 32,833 patient-years). In another report based on a hospital cohort of 1935 patients with CD involving the small bowel [73], the cumulative risk of SBC in CD was estimated to be 2 (95 % CI, 0–8) and 22 (95 % CI, 7–64) per 1000 patients after 10 and 25 years of follow-up, respectively. In a recent large, prospective, cohort study of SBC in CD [74], five SBCs were found in 8222 patients with small bowel CD (either alone or associated with colonic CD) during a median follow-up of 35 months (range, 29–40 months). The incidence rates of SBC were 0.235 per 1000 patient-years (95 % CI, 0.076–0.547) among patients with small bowel CD and 0.464 per 1000 patient-years (95 % CI, 0.127–1.190) among those with small bowel CD for 8 years. This accounted for approximately 30 % of the risk of CRC in patients with CD of the colon. Patients with small bowel CD and small bowel CD for 8 years had an SBC SIR of 34.9 (95 % CI, 11.3–81.5) and 46.0 (95 % CI, 12.5–117.8), respectively.
Dossett et al. [75] summarized the features of 154 cases of SBC in CD reported in Europe and America. SBC occurred more frequently in males than in females (M:F = 2.4:1). The age at diagnosis ranged from 21 to 86 years (mean age, 51.3 years), and the average duration of CD was 24.5 years (range, 0–45 years) [75]. CD patients who developed SBC were younger than those with de novo cancers, which occurred mainly in patients 60–69 years of age [76, 77]. Ileal tumors occurred at a rate of 75 %. The presence of tumor in previously bypassed segments of the intestine was 20.3 %. Obstruction was the most common manifestation (76 %), whereas hemorrhage, fistula, and perforation were present in 3.9 %, 3.9 %, and 5.4 % of the cases, respectively. The majority of the diagnoses were made at the time of surgery (35.4 %) or postoperatively (61.5 %). Only 3.1 % of the cases were diagnosed preoperatively.
Thus, patients with CD are also at risk of CA-SBC, albeit the incidence is lower than that of CRC or ARC. However, while the incidence of SBC is low in the general population, it is significantly higher in patients with CD (RR = 27) [78]. Nonetheless, since <1 % of patients with CD will develop SBC and given the similarity of SBC findings and those of CD lesions on some examinations, the diagnosis of SBC in CD patients can be very challenging [79, 80]. Moreover, the prognosis of CD patients with SBC is poor. In the study of Elriz et al. [74], four of the five patients with SBC, diagnosed at an advanced stage by resected specimens, died within 3 years. In the study of Dossett et al. [75], based on 154 cases of SBC in CD reported in Europe and America, the survival rates after 1 and 2 years were 49.6 % and 27 %, respectively. Even in SBC in patients with non-CD, diagnosis was made at a late stage because of limited and insensitive examinations or vague presenting symptoms. Consequently, these patients have a worse prognosis, with a reported 5-year survival of 26 % [81, 82].
In all reports on the incidence of CRC, patients with CD were diagnosed with a more advanced stage of disease than those with UC or no IBD: either Dukes’ B or C; stage 3, with lymph node involvement; stage 4; or T2 or T3 with a positive N number [5, 56–59]. Furthermore, in CD, tumors with mucinous components or poorly differentiated adenocarcinomas are not uncommon, even in CA-CRC in CD, regardless of the site of anatomic involvement [26]. In conclusion, the prognosis of CA-CRC in patients with CD may depend on the cancer stage at diagnosis and the pathological features, as in UC; but in patients with long-standing CD, the possibility of concomitant cancer should be considered even in sites of strictureplasty [82–84].
3.4 Chemoprevention of CAC
The results of studies on the risk factors CA-CRC [16–21] suggest that long-standing and continuous inflammation lead to carcinogenesis and in turn CA-CRC. It is also clear that long-term failure to achieve remission from active colitis is a risk factor for CA-CRC [19, 22]. Chronic inflammation may contribute to carcinogenesis by generating a favorable microenvironment for cancer development and progression. Increased concentrations of inflammatory cytokines or mediators, such as reactive oxygen and nitrogen species or cyclooxygenase-2-associated prostaglandins, and alterations of DNA, RNA, proteins, or lipids have been invoked as events leading to tumor formation. For example, the p53 tumor suppressor gene is mutated in the inflamed mucosa, and expression of the gene was shown to correlate with the intensity of inflammation and with the degree of dysplasia [85]. Thus, it may be that strategies aimed at reducing the inflammation in IBD may also decrease the risk of CA-CRC. Several approaches to achieving this goal are described in the following.
3.4.1 Probiotics
The human gut microflora, comprising some 100 trillion microbial organisms, is critical in maintaining host health, both in the gastrointestinal tract itself and systemically, through the absorption of metabolites [86]. Recent studies have implicated specific strains of bacteria in the regulation of intestinal homeostasis, by delivering regulatory signals to the epithelium, to the mucosal immune system, and to the neuromuscular activity of the gut [87, 88]. However, some commensal and pathogenic organisms belonging to the human enteric microbiome contribute to the pathogenesis of IBD and CRC. Therefore, the use of probiotic bacteria to manipulate gut bacterial composition and local metabolite production has been explored as a therapeutic intervention to prevent CRC. Probiotics are live microbial food supplements with positive effects on host health. However, the efficacy of probiotics in this setting is unclear, and studies in mice have yielded controversial results [88–90]. To date, there have been no proper evaluations in humans.
3.4.2 Aminosalicylate
Patients with IBD are commonly administered 5-aminosalicylate (5-ASA) as maintenance therapy, which in in vitro studies was shown to have antineoplastic properties by inhibiting the nuclear kappa-B pathway involved in tumor progression [91]. However, the evidence for a potential chemoprophylactic effect of 5-ASA is contradictory. In 2005, Velayos et al. [92] reported a meta-analysis of nine studies examining the effect of 5-ASA in preventing CA-CRC in IBD. The pooled analysis revealed a protective effect of 5-ASA use on the risk of CA-CRC (odds ratio = 0.51; 95 % CI, 0.37–0.69). However, a chemopreventive effect of 5-ASA has not been confirmed in case–control and population-based studies [93–95]. In a recent meta-analysis, Nguyen et al. [96] reported a pooled adjusted odds ratio of 0.95 (95 % CI, 0.66–1.38) for CA-CRC in patients with IBD treated with 5-ASA, nor did 5-ASA seem to have a protective effect in preventing CA-CRC in IBD. However, long-standing mild colitis, which could be maintained by 5-ASA administration alone, without aggressive therapy such as corticosteroids, immunomodulators, or biologics, could increase the risk of CA-CRC [15], by maintaining a state of chronic inflammation without complete remission or mucosal healing.
3.4.3 Immunomodulators
An increasing number of IBD patients are being treated with the thiopurine drugs azathioprine and 6-mercaptopurine, based on the consideration that aggressive therapy can induce complete remission and may therefore have a preventive effect. However, data on the potential chemopreventive effect of thiopurines in IBD are conflicting. In the CESAME study [58], nearly half of the 19,484 IBD patients had been exposed to thiopurines; among current users, the adjusted hazard ratio (HR) for CA-CRC was 0.57 (95 % CI, 0.24–1.32), thus ruling out a significant protective effect of thiopurine use on CA-CRC risk in the general IBD population. However, in a subanalysis confined to IBD patients with long-standing extensive colitis, current treatment with thiopurines reduced the risk of advanced CRC significantly (HR 0.28; 95 % CI, 0.09–0.89). In a Dutch cohort study, van Schaik et al. [97] estimated the effect of thiopurines on the risk of CA-CRC in an IBD cohort of 2578 IBD patients, of whom 770 had been exposed to thiopurines. They found that thiopurine exposure decreased the risk of advanced CA-CRC significantly (adjusted HR = 0.10; 95 % CI, 0.01–0.75). However, in a Danish, nationwide, population-based study, Pasternak et al. [98] found no effect of thiopurine on the occurrence of CA-CRC among 43,969 IBD patients, of whom 12 % had been exposed to thiopurines (adjusted RR = 1.00; 95 % CI, 0.61–1.63). Similar results were reported in another large population-based study from the United Kingdom [99].
3.4.4 Biologics
Recent data from models of experimental colitis have demonstrated the tumor-promoting effect of tumor necrosis factor (TNF)-α [100]; but, only a few studies have evaluated the effect of new biological treatments, such as anti-TNF-α, on the risk of CA-CRC, as they have not been used long enough relative to the latency of CA-CRC. In a Dutch nested case–control study, Baars et al. [22] evaluated the risk factors for CA-CRC by comparing 173 CA-CRC patients with 393 non-CRC patients with IBD. They found that the use of anti-TNF-α was a protective factor for the development of CA-CRC (odds ratio = 0.09; 95 % CI, 0.01–0.68). In a Danish nationwide population-based cohort study, the risk of CA-CRC was compared between patients treated or not with anti-TNF-α. There was no correlation between anti-TNF-α and the prevalence of CA-CRC (adjusted RR = 1.06; 95 % CI, 0.33–3.40) [101]. Given that anti-TNF-α may be both tumor promoting and tumor preventing, further studies are needed to confirm its efficacy as a chemopreventive agent.
3.5 Prophylactic Efficacy of Proctocolectomy
3.5.1 Proctocolectomy in Patients with UC
Surveillance colonoscopy for CA-CRC has been standardized, and its use in patients with long-standing IBD (≥8–10 years) has been widely advocated [7–9, 11, 23, 24]. Dysplasia or cancer found in surveillance is an absolute indication for proctocolectomy in patients with UC. Although it remains unclear whether proctocolectomy can decrease the incidence of CA-CRC in UC and improve the prognosis of these patients, the 5-year survival rate was higher in patients who underwent surveillance (87 %) than in those who did not (55 %) [43]. In Japan, Hata et al. [13] suggested that surveillance would allow the detection of CA-CRC at an earlier stage. In a recent review and meta-analysis of the incidence of CA-CRC, the risk of UC patients developing CRC was found to have decreased steadily over the last six decades [102]. Whether these improvements can be attributed to surveillance colonoscopy and prophylactic proctocolectomy remains to be determined.
3.5.2 Proctocolectomy in Patients with Anorectal CD
In patients at risk of SBC, prophylactic resection of the small intestine is contraindicated by its absolute necessity for nutrient absorption, although total parenteral nutrition therapy can be considered. However, in patients with anorectal CD, prophylactic proctectomy may decrease the incidence of ARC. As discussed in Sect. 3.3, ARC is rarely detected in its early stage owing to the difficulty of proper surveillance and accurate diagnosis. Furthermore, ostomy diversion of anorectal CD increases the risk of ARC [64, 65, 69]. However, in patients with severe perianal disease with stricture, in whom both medical management and local surgical treatment have failed, the first surgical option is a diverting colostomy or ileostomy. If these do not provide sufficient relief, the final option is proctectomy, performed in 10–20 % of patients with perianal CD [103, 104]. Proctectomy generally consists of either a low Hartmann’s procedure or a complete proctectomy with sphincter resection. The disadvantages of Hartmann’s procedure include the risk of ARC in the residual distal rectum. This can cause persisting complaints of Crohn’s disease, and anastomotic breakdown will result in a presacral abscess draining and fistulizing through the perineum [105–108]. Therefore, in our institution, complete proctectomy is preferred when the stricturing anorectal lesion, regardless of the presence of a fistula tract, cannot be improved by any treatment. In our experience, proctectomy for these lesions improves not only the severe symptoms of anorectal lesions but also the incidence of ARC. However, an as yet unresolved problem is that the perineal wound after proctocolectomy heals poorly and there is a high incidence of persistent sinus in patients with CD [109, 110]. Long-term cohort studies, performed after several decades, are still needed to determine the outcomes of these patients.
References
1.
Eaden JA, Abrams KR, Mayberry JF (2001) The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut 48:526–535PubMedCentralPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree